Calculate the number of milliliters of 0.753 M KOH required to precipitate all of the Pb2* ions
Fantastic news! We've Found the answer you've been seeking!
Question:
Calculate the number of milliliters of 0.753 M KOH required to precipitate all of
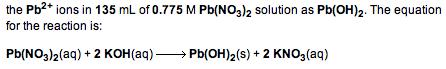
Related Book For 

Posted Date:




