Question: Calculate the time required to achieve 90% conversion for second order reaction in a constant volume batch reactor, if the value of k is 104s1
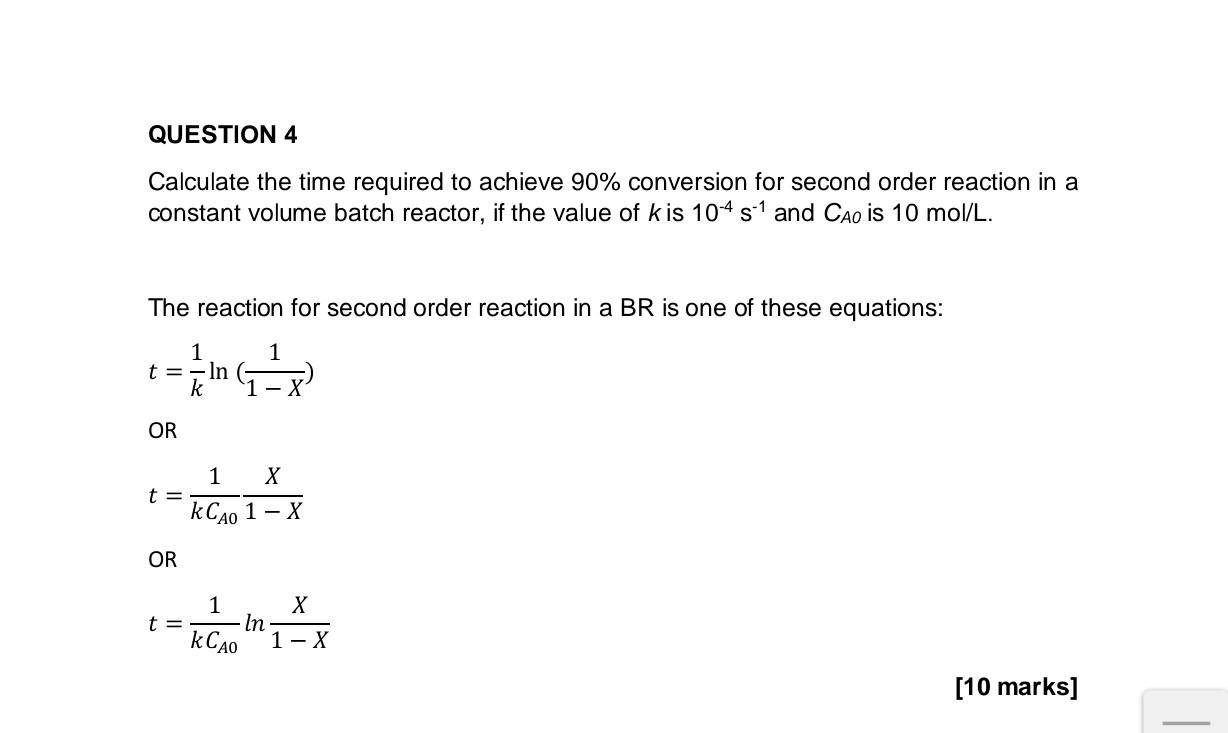
Calculate the time required to achieve 90% conversion for second order reaction in a constant volume batch reactor, if the value of k is 104s1 and CAO is 10mol/L. The reaction for second order reaction in a BR is one of these equations: t=k1ln(1X1) OR t=kCA011XX OR t=kCA01ln1XX
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


