Question: a. Calculate the EMF of cells A through F at standard states (Ecell) and non-standard states (Ecell) using Nernst equation. Fill in Table-1 with

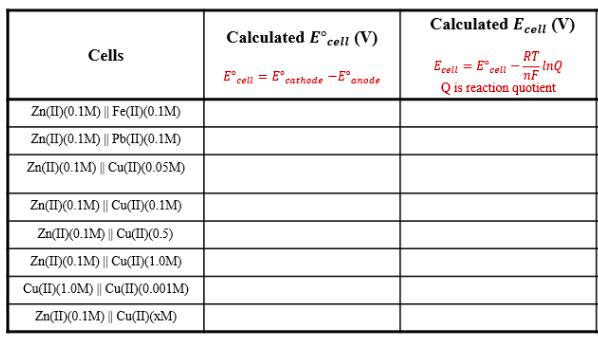
a. Calculate the EMF of cells A through F at standard states (Ecell) and non-standard states (Ecell) using Nernst equation. Fill in Table-1 with your answers. Calculated E coell (V) Calculated Ec cell (V) Cells RT Ecell = E cell InQ = E E cell nF cathode anode Q is reaction quotient Zn(I)(0.1M) || Fe(I)(0.1M) Zn(II)(0.1M) || Pb(II)(0.1M) Zn(II)(0.1M) || Cu(II)(0.05M) Zn(I)(0.1M) || Cu(II)(0.1M) Zn(IX0.1M) || Cu(II(0.5) Zn(I)(0.1M) || Cu(I)(1.0M) Cu(I)(1.0M) || Cu(II(0.001M) Zn(I)(0.1M) || Cu(II)(xM)
Step by Step Solution
3.47 Rating (154 Votes )
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


