Question: Calculating equilibrium concentrations when the net reaction proceeds forward Consider mixture B, which will cause the net reaction to proceed forward. The change in concentration,
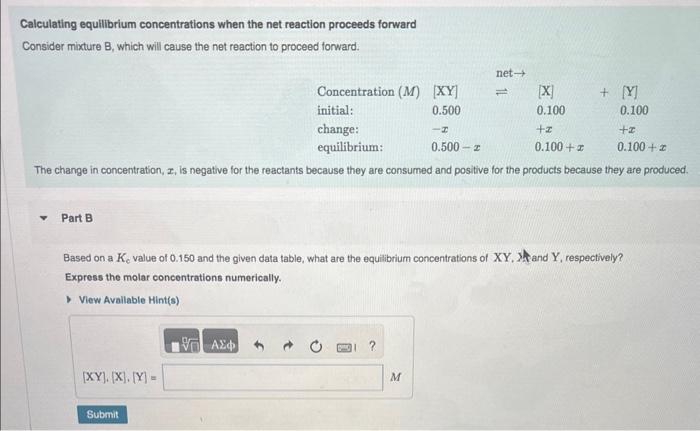
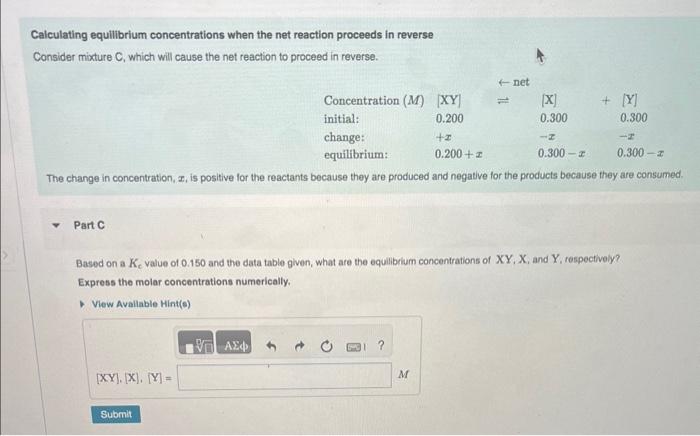
Calculating equilibrium concentrations when the net reaction proceeds forward Consider mixture B, which will cause the net reaction to proceed forward. The change in concentration, x, is negative for the reactants because they are consumed and positive for the products because they are produced Part B Based on a Kc value of 0.150 and the given data table, what are the equilibrium concentrations of XY. Shand Y, respectively? Express the molar concentrations numerically. Calculating equilibrium concentrations when the net reaction proceeds in reverse Consider mixture C, which will cause the net reaction to proceed in reverse. The change in concentration, x, is positive for the reactants because they are produced and negatve for the products because they are consumed. Part C Based on a Kc value of 0.150 and the data table given, what are the equilibrium concentrations of XY,X, and Y, respectively? Express the molar concentrations numerically
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


