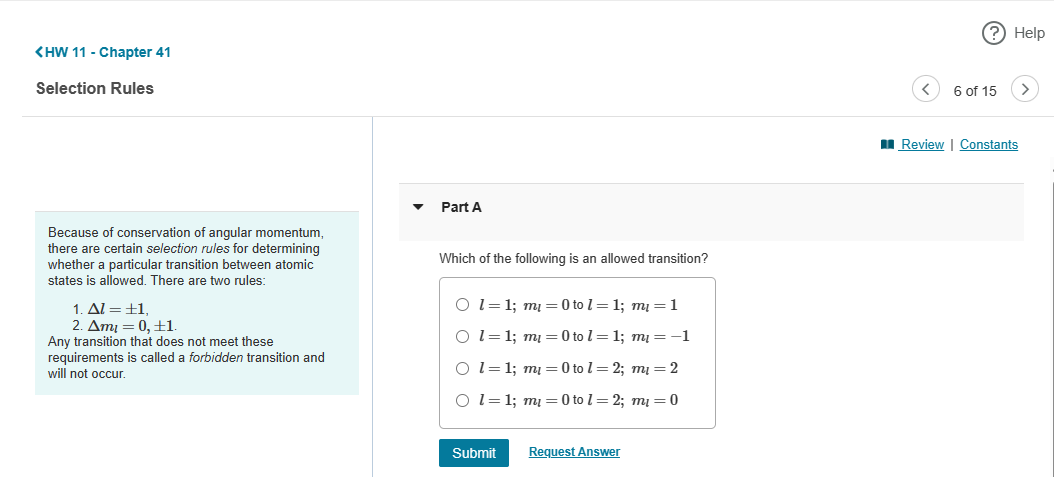
Question: Calculus Based Physics 3 Chapter 41 Quantum Mechanics 2 Question 6 ? Help Review | Constants Part A Because of conservation of angular momentum, there
Calculus Based Physics 3 Chapter 41 Quantum Mechanics 2 Question 6
? Help
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


