Question: can anyone explain how to find this answer Consider the heating curve provided for 1.00 mole of a substance that begins as a solid at
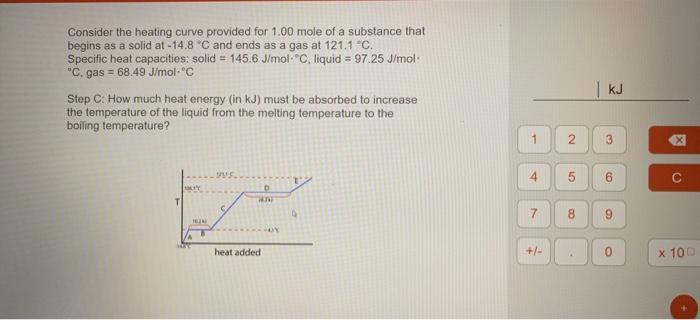
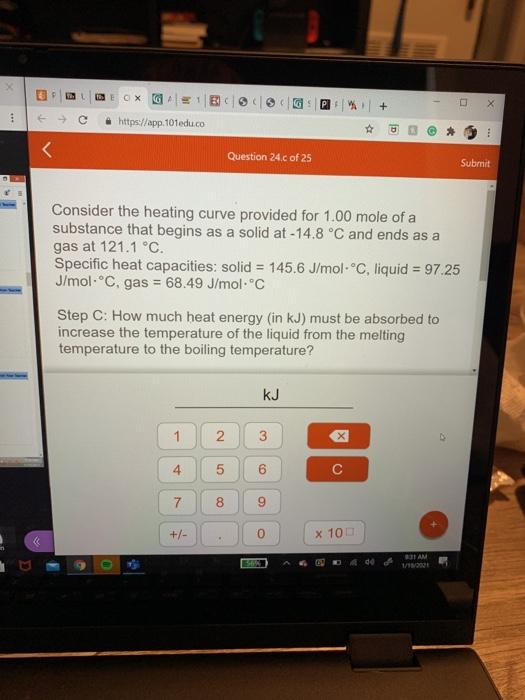
Consider the heating curve provided for 1.00 mole of a substance that begins as a solid at -14.8 C and ends as a gas at 121.1-C. Specific heat capacities: solid = 145.6 J/mol. Cliquid = 97 25 J/mol "C. gas = 68.49 J/molC Step C: How much heat energy (in kJ) must be absorbed to increase the temperature of the liquid from the melting temperature to the boiling temperature? E 1 2 3 x 4 6 D T 7 8 9 heat added +/- 0 X 100 G B OX GALE GP + https://app.101edu.co
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


