Question: Can I get help with question 1 & 2 pls. Thank you! Question 1 (A) Draw the VSEPR predicted shape (include all lone pairs and
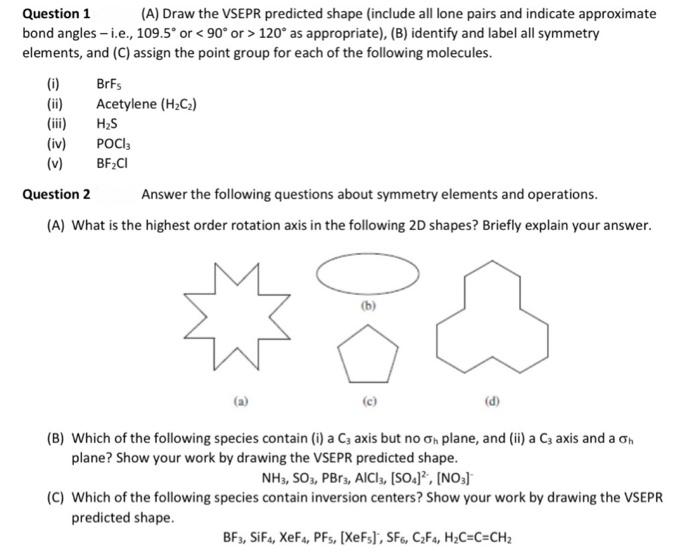
Question 1 (A) Draw the VSEPR predicted shape (include all lone pairs and indicate approximate bond angles - i.e., 109.5 or 120 as appropriate), (B) identify and label all symmetry elements, and (C) assign the point group for each of the following molecules. (0) BrFs Acetylene (H2C2) HAS POCI BF,CI Question 2 Answer the following questions about symmetry elements and operations. (A) What is the highest order rotation axis in the following 2D shapes? Briefly explain your answer. (B) Which of the following species contain (i) a C; axis but no on plane, and (ii) a C3 axis and a on plane? Show your work by drawing the VSEPR predicted shape. NH3, 503, PBr3, AlCl3, [S04, (NO3] (C) Which of the following species contain inversion centers? Show your work by drawing the VSEPR predicted shape. BF3, SiF4, XeF, PFs, [XeFs], SF6, CzF4, H2C=C=CH2
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


