Question: Can I get some help witb numbers 3, 4, and 5 please? + 3. The following elementary reaction has been proposed as the rate-determining step

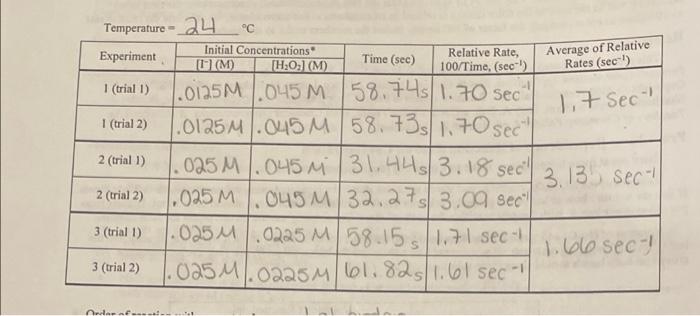
+ 3. The following elementary reaction has been proposed as the rate-determining step for this reaction system: H2O, +1"+H HOI + H2O Is this reaction consistent with your results? Explain. 4. In these experiments, we used a consistent (and large) amount of sulfuric acid so that we could ignore the possible effect of [H"). Propose an experiment similar to those you did but that would allow you to test the reaction system's dependence on [H") 5. Based on the proposed rate-determining step, what would you expect the order of the reaction to be with respect to H"? 28 Initial Concentrations (H:0:1 (M) Average of Relative 1,7 Sect Temperature - 24 C Relative Rate, Experiment [tr](M) Time (sec) 100/Time, (sec) Rates (sec) 1 (trial 1) .0125M .045 M 58.745 1.70 sec 1 (trial 2) .0125M .045 M 58.735 1.70 sec 2 (trial 1) 025 M .045 m 31,445 3.18 sec" 3.135 sec 2 (trial 2) ,025 M .045 M 32.278 3.09 sec" 3 (trial 1) .025 M 0225 M 58.15 1.7 sec - 1.blo sec) 3 (trial 2) .025 M.0225 M 61.825 1.6 sec - M P S . ndan
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


