Question: Can I please get some help on these very easy question Ill make sure to upvote thank you NOTE: This is a multi-part question. Once
Can I please get some help on these very easy question Ill make sure to upvote thank you
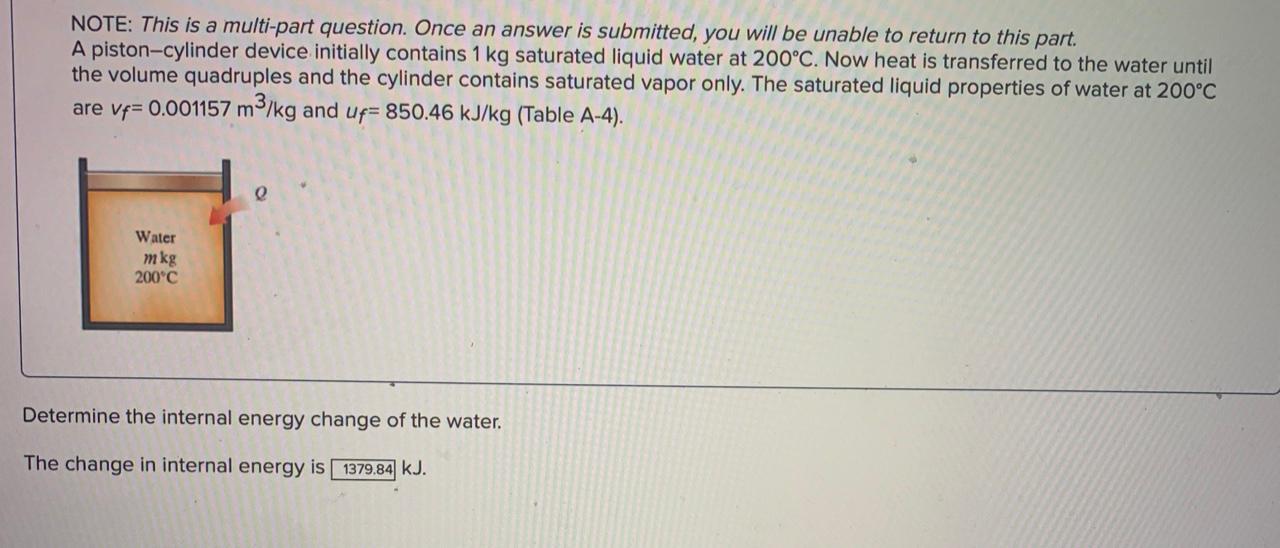
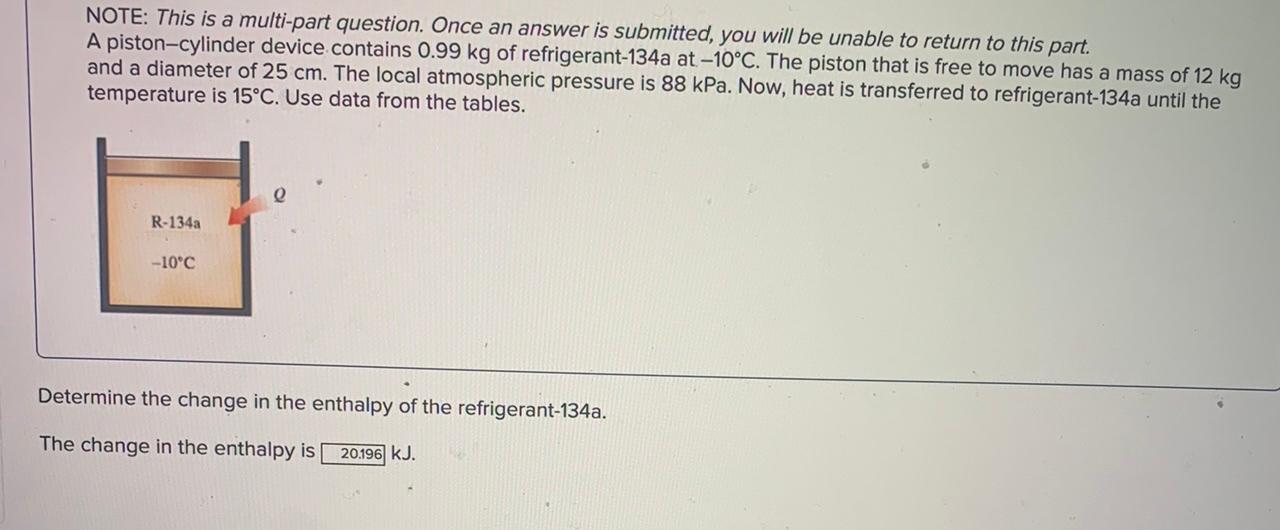
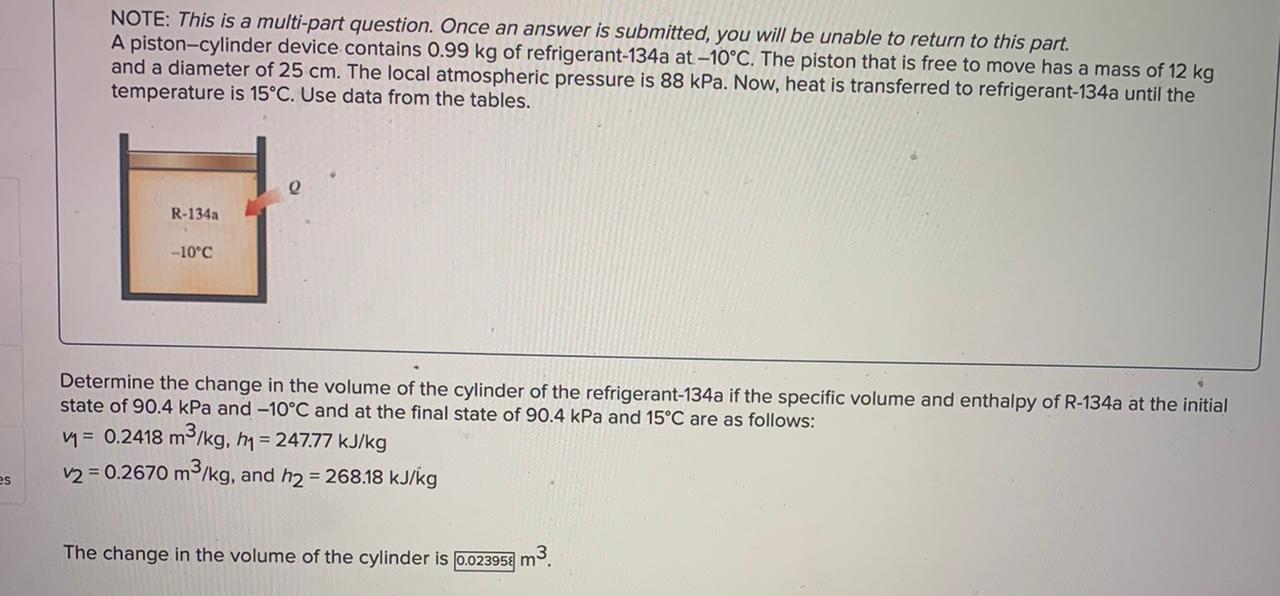
NOTE: This is a multi-part question. Once an answer is submitted, you will be unable to return to this part. A piston-cylinder device initially contains 1 kg saturated liquid water at 200C. Now heat is transferred to the water until the volume quadruples and the cylinder contains saturated vapor only. The saturated liquid properties of water at 200C are vf= 0.001157 m3/kg and up= 850.46 kJ/kg (Table A-4). e Water mkg 200C Determine the internal energy change of the water. The change in internal energy is 1379,84 kJ. NOTE: This is a multi-part question. Once an answer is submitted, you will be unable to return to this part. A piston-cylinder device contains 0.99 kg of refrigerant-134a at -10C. The piston that is free to move has a mass of 12 kg and a diameter of 25 cm. The local atmospheric pressure is 88 kPa. Now, heat is transferred to refrigerant-134a until the temperature is 15C. Use data from the tables. R-134a -10C Determine the change in the enthalpy of the refrigerant-134a. The change in the enthalpy is 20.196 kJ. NOTE: This is a multi-part question. Once an answer is submitted, you will be unable to return to this part. A piston-cylinder device contains 0.99 kg of refrigerant-134a at -10C. The piston that is free to move has a mass of 12 kg and a diameter of 25 cm. The local atmospheric pressure is 88 kPa. Now, heat is transferred to refrigerant-134a until the temperature is 15C. Use data from the tables. e R-134a -10C Determine the change in the volume of the cylinder of the refrigerant-134a if the specific volume and enthalpy of R-134a at the initial state of 90.4 kPa and -10C and at the final state of 90.4 kPa and 15C are as follows: m = 0.2418 m3/kg, h1 = 247.77 kJ/kg v2 = 0.2670 m3/kg, and h2 = 268.18 kJ/kg es The change in the volume of the cylinder is 0.023958 m3
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


