Question: can please help me solve me solve ghe lower half on the paper. im not understanding how to solve it T Rates of Chemical Reactions:
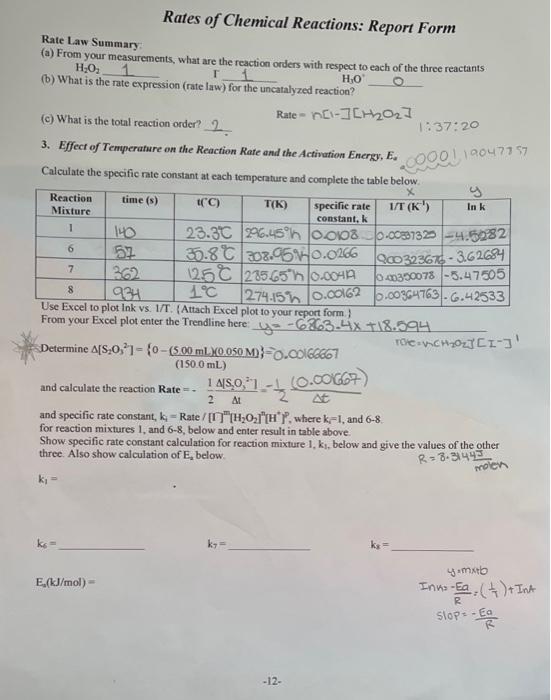
T Rates of Chemical Reactions: Report Form Rate Law Summary (a) From your measurements, what are the reaction orders with respect to each of the three reactants H:02 1 HO (b) What is the rate expression (rate law) for the uncatalyzed reaction? Rate - nd-]CH2022 (c) What is the total reaction order? 2 3. Effect of Temperature on the Reaction Rate and the Activation Energy, E. Calculate the specific rate constant at each temperature and complete the table below. time (s) (C) T(K) specific rate 1/T (K) 1:37:20 10047757 0001 Reaction Mixture y In k constant, 1 6 900323675 - 362684 7 8 140 23.30 206.45n JO008 0.00837320 -4.5082 52 5.80 308.56 0.0266 1250 285657 0.0041 040350078 -5.47505 934 1c 274.15 0.00162 0.00364763-G.42533 Use Excel to plot Ink vs. 1/T. (Attach Excel plot to your report form From your Excel plot enter the Trendline here yo-643-4x 118.594 roiech2021C1-3) Determine A[3,0,1 - {0-(500 mL.X0.050 M}-O.C01666667 (150.0 mL) and calculate the reaction Rate - 15.0, 1.- At and specific rate constant, k = Rate / "H2021"*'where k=1, and 6-8 for reaction mixtures 1 and 6-8, below and enter result in table above. Show specific rate constant calculation for reaction mixture 1, k, below and give the values of the other three. Also show calculation of E. below. 1.-0.00667) 2 AL R=3-31443 molen ki = ke ky 4.mtb E.(kJ/mol) - Inno-Ea, ( ) + Int R slope -Ear -12
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


