Question: Can some one break this down, and explain how what is happening ? Thank you The acid dissociation constant, Ka, for benzoic acid, C6H5COOH, is
Can some one break this down, and explain how what is happening ?
Thank you
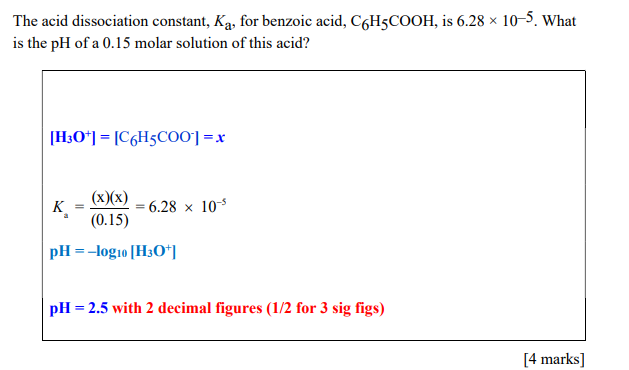
The acid dissociation constant, Ka, for benzoic acid, C6H5COOH, is 6.28105. What is the pH of a 0.15 molar solution of this acid? [H3O+]=[C6H5COO]=x Ka=(0.15)(x)(x)=6.28105pH=log10[H3O+] pH=2.5 with 2 decimal figures ( 1/2 for 3 sig figs)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


