Question: Can someone double check my work for this question. Having to find the quantum numbers (nx, ny,and nz) in a cubic quantum box is throwing

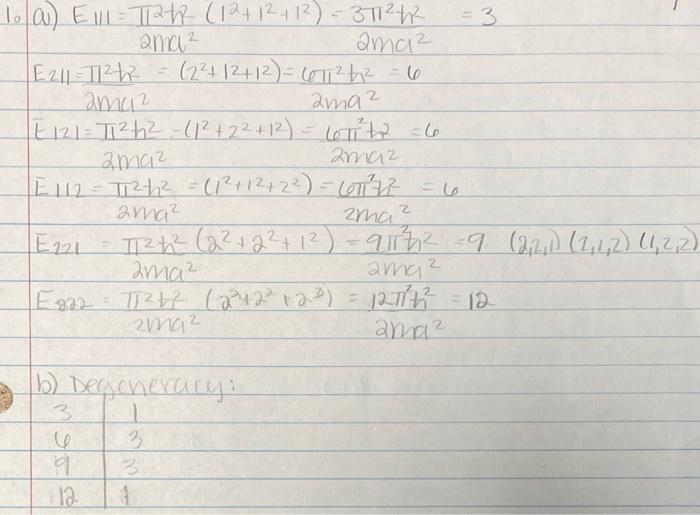
1. Consider a particle in a cubic quantum mechanical box.(a) Identify the values of the quantum numbers (nx,ny,nz) that determine the four lowest energy levels. (b) Determine the degeneracy of each level. a) E11=2ma22+h2(12+12+12)=2ma232h2=3 E211=2ma22h2=(22+2+12)=2ma262h2=6 E2=2ma22h2(12+22+12)=2ma262h2=6 E12=2ma22h2=(12+12+22)=2ma262h2=6 E221=2ma222(22+22+12)=2ma292h2=9 E822=2ma22+h2(22+22+23)=2ma21222=12
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


