Question: can someone explain this is depth. why does x=10? Titration of Histidine with NaOH pH=pKa+[HA][A]log7=6x=10+logX One in every 11 is protonated at pH7 Histidine serves
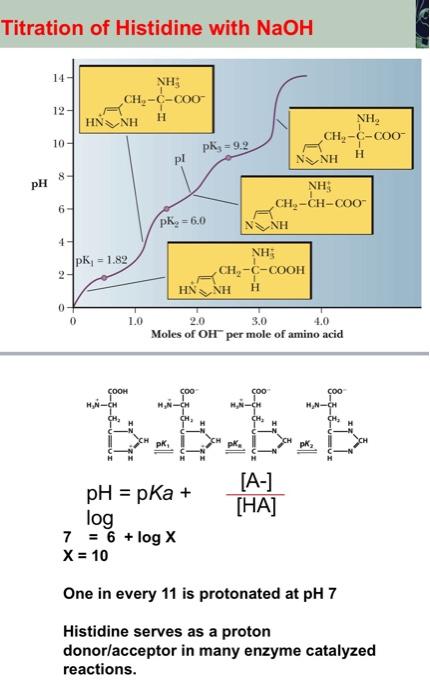
Titration of Histidine with NaOH pH=pKa+[HA][A]log7=6x=10+logX One in every 11 is protonated at pH7 Histidine serves as a proton donor/acceptor in many enzyme catalyzed reactions
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


