Question: Can someone explain what's going on here for part d in the last step? I don't understand where the numbers to calcualte Y(o) came from.
Can someone explain what's going on here for part d in the last step? I don't understand where the numbers to calcualte Y(o) came from.
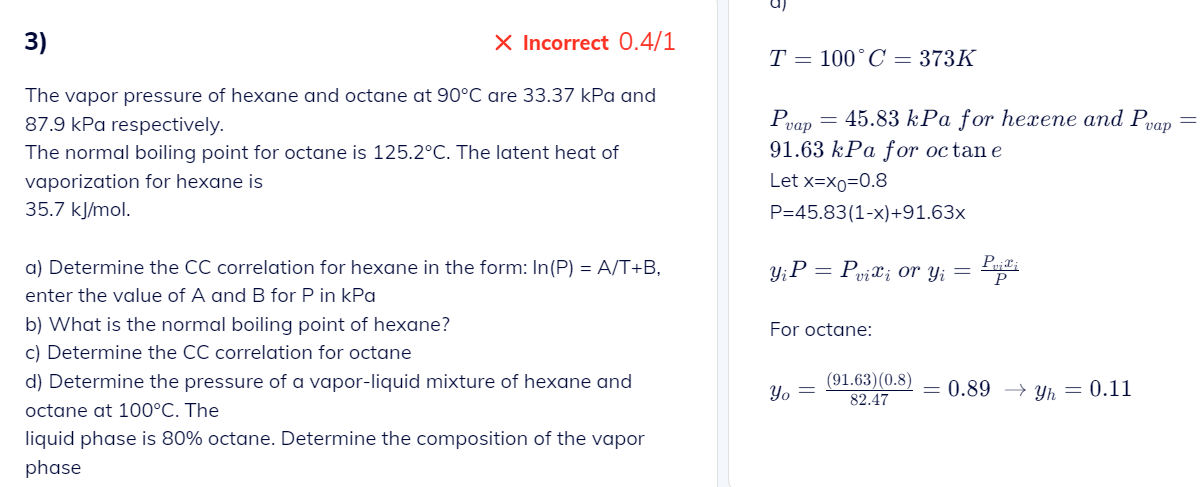
3) Incorrect 0.4/1 The vapor pressure of hexane and octane at 90C are 33.37kPa and 87.9kPa respectively. The normal boiling point for octane is 125.2C. The latent heat of vaporization for hexane is 35.7kJ/mol. a) Determine the CC correlation for hexane in the form: ln(P)=A/T+B, enter the value of A and B for P in kPa b) What is the normal boiling point of hexane? c) Determine the CC correlation for octane d) Determine the pressure of a vapor-liquid mixture of hexane and octane at 100C. The liquid phase is 80% octane. Determine the composition of the vapor phase T=100C=373K Pvap=45.83kPa for hexene and Pvap= 91.63kPa for oc tane Let x=x0=0.8 P=45.83(1x)+91.63x yiP=Pvixi or yi=PPvixi For octane: yo=82.47(91.63)(0.8)=0.89yh=0.11
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


