Question: Can someone help me fully understand and answer this question? I previously got it wrong. The correct answer will be upvoted, thanks A bomb calorimeter
Can someone help me fully understand and answer this question? I previously got it wrong. The correct answer will be upvoted, thanks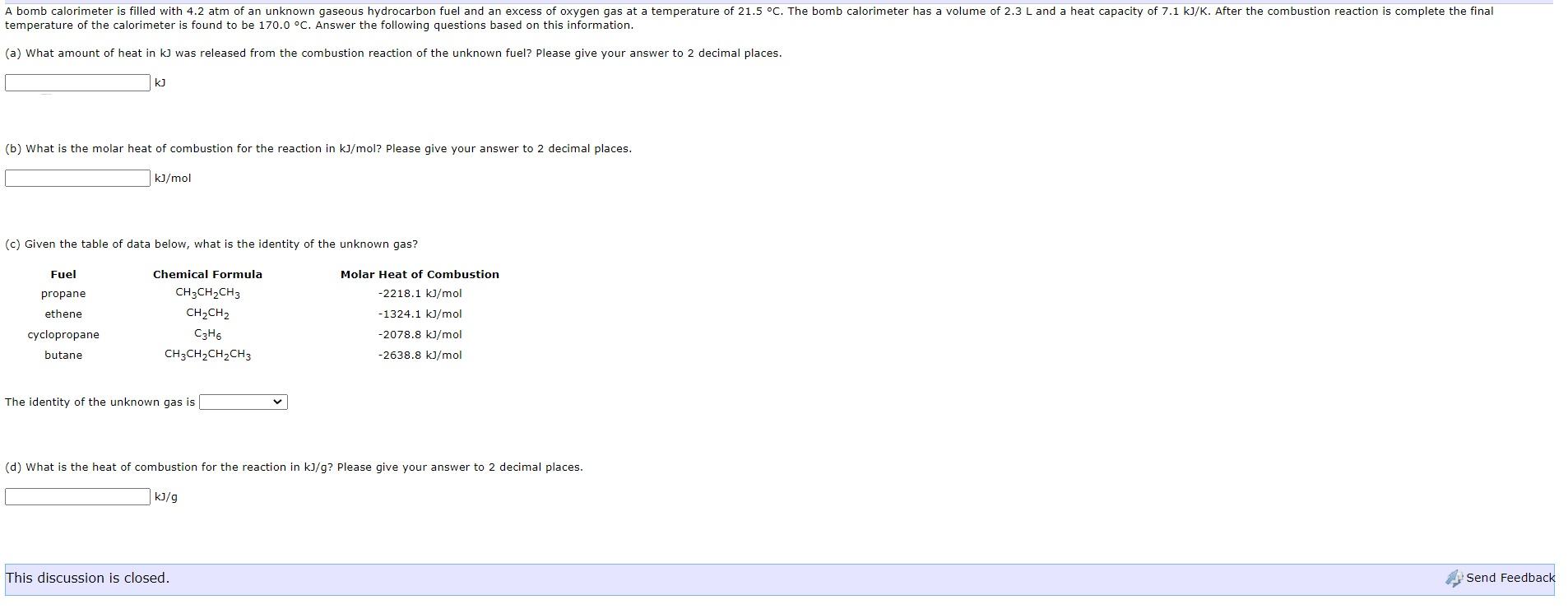
A bomb calorimeter is filled with 4.2 atm of an unknown gaseous hydrocarbon fuel and an excess of oxygen gas at a temperature of 21.5 C. The bomb calorimeter has a volume of 2.3 L and a heat capacity of 7.1 kJ/K. After the combustion reaction is complete the final temperature of the calorimeter is found to be 170.0 C. Answer the following questions based on this information. (a) What amount of heat in k] was released from the combustion reaction of the unknown fuel? Please give your answer to 2 decimal places. ku (b) What is the molar heat of combustion for the reaction in kJ/mol? Please give your answer to 2 decimal places. kJ/mol (c) Given the table of data below, what is the identity of the unknown gas? Fuel Molar Heat of Combustion -2218.1 kJ/mol Chemical Formula CH3CH2CH3 CH2CH2 C3H6 CH3CH2CH2CH3 propane ethene cyclopropane butane -1324.1 kJ/mol -2078.8 kJ/mol -2638.8 kJ/mol The identity of the unknown gas is (d) What is the heat of combustion for the reaction in kJ/g? Please give your answer to 2 decimal places. kJ/g This discussion is closed. Send Feedback
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


