Question: Can someone help me solve 1-7 Trial 1- 175 seconds Trial 2- 95 seconds Trial 3- 66 seconds Trial 4- 32 seconds Introduction This experiment

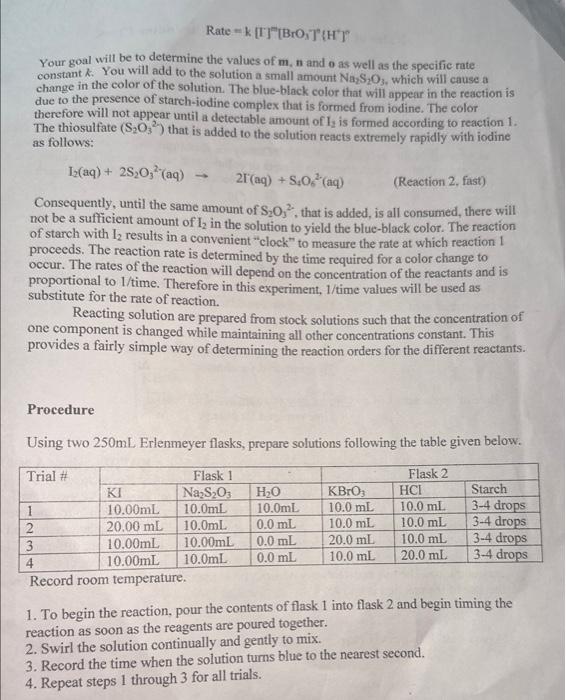
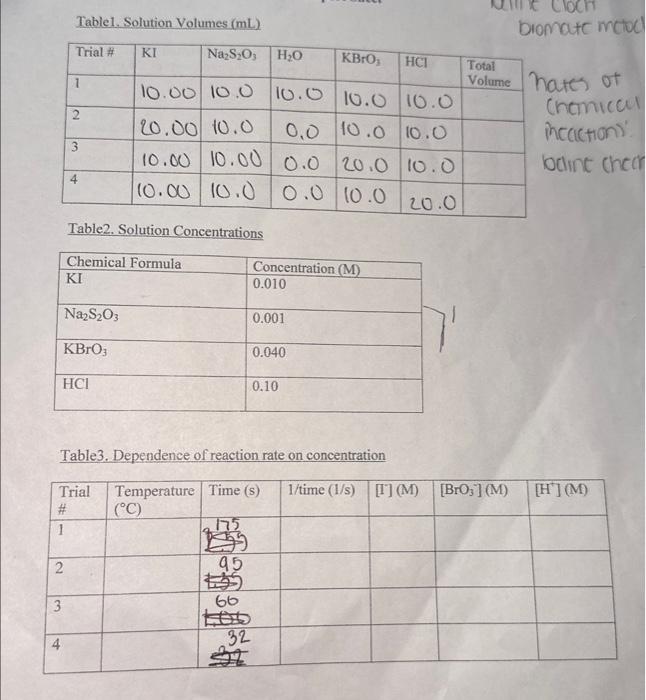

Introduction This experiment will investigate a reaction occurring at different rafes ai a resuit of varying the concentration of reactants. Kinetic is the area of chemistry that deals with the studying of the rates of reactions. There are four factors that control reaction nites in solution. These include the nature of the reactants, the coneentration of the reactants, the molecules obtain enoughen, and the use of catalysts. Reactions will occur when reactant one of the factors above afigy to interact or collide with other reactint molecules. Each other. Consider the general reaction scheme: A+BC+D The rate of the reaction can be measured by observing the rate at which the reactants A or B disappear or the rate at which products C or D appear. Experimentally, the change in concentration of A,B,C, and D with time can be measured. The rate of reaction can be expressed as: Rate of disappearance of A= Change in concentration of A=[A] Time required for change t The rate law for the general reaction scheme is given as Rate =k[A][[B]y, where A and B are the molar concentration of reactants, and x and y are orders of the reaction with respect to A and B.k is the rate constant. Adding x and y together will give the overall order of the reaction. One method of determining the order of the reaction is to observe the changes of the reaction as reactant concentrations are varied. The stoichiometry and the order of the reaction have no dependence on each other and usually are different. The rate constant, k, has a definite value for a given reaction and is independent of the concentration. The rate constant only depends on temperature and may be calculated once the rate of the reaction is known. In this experiment, we will study the following chemical reaction: 6I(aq)+BrO3(aq)+6H+(aq)3I2(aq)+Br(aq)+3H2O (Reaction 1 , slow) The rate of reaction 1 depends on the concentration of I,BrO3 and H+in solution. The rate law to be determined is of the form Your goal will be to determine the values of m,n and 0 as well as the specific rate constant k. You will add to the solution a small amount Na2S2O3, which will cause a change in the color of the solution. The blue-black color that will appear in the reaction is due to the presence of stareh-iodine complex that is formed from iodine. The color therefore will not appear until a detectable amount of 12 is formed according to reaction 1 . The thiosulfate (S2O32) that is added to the solution reacts extremely rapidly with iodine as follows: I2(aq)+2S2O32(aq)2r2(aq)+S4O62(aq) (Reaction 2, fist) Consequently, until the same amount of S2O32, that is added, is all consumed, there will not be a sufficient amount of I2 in the solution to yield the blue-black color. The reaction of starch with I2 results in a convenient "clock" to measure the rate at which reaction 1 proceeds. The reaction rate is determined by the time required for a color change to occur. The rates of the reaction will depend on the concentration of the reactants and is proportional to 1/ time. Therefore in this experiment, 1/ time values will be used as substitute for the rate of reaction. Reacting solution are prepared from stock solutions such that the concentration of one component is changed while maintaining all other concentrations constant. This provides a fairly simple way of determining the reaction orders for the different reactants. Procedure Using two 250mL Erlenmeyer flasks, prepare solutions following the table given below. Record room temperature. 1. To begin the reaction, pour the contents of flask 1 into flask 2 and begin timing the reaction as soon as the reagents are poured together. 2. Swirl the solution continually and gently to mix. 3. Record the time when the solution turns blue to the nearest second. 4. Repeat steps 1 through 3 for all trials. Table1. Solution Volumes (mL) Table2. Solution Concentrations Table3. Dependence of reaction rate on concentration Calculations 1. Calculate the exact concentrations of KI,Na2S2O3,KBrO3 and HCl. 2. Using the experimental data collected, evaluate the exponents of the rate law expression. 3. Write out the complete rate law expression for reaction 1 . 4. What is the overall order of the reaction? 5. Calculate the rate constant, k, for each trial by substituting the concentration of r, BrO3, and H+and the values of m,n, and o into rate law expression. 6. Calculate the average rate constant. (Show proper units for the rate constant) 7. If you want to double the rate of the iodine clock reaction, what experimental changes must be made? Introduction This experiment will investigate a reaction occurring at different rafes ai a resuit of varying the concentration of reactants. Kinetic is the area of chemistry that deals with the studying of the rates of reactions. There are four factors that control reaction nites in solution. These include the nature of the reactants, the coneentration of the reactants, the molecules obtain enoughen, and the use of catalysts. Reactions will occur when reactant one of the factors above afigy to interact or collide with other reactint molecules. Each other. Consider the general reaction scheme: A+BC+D The rate of the reaction can be measured by observing the rate at which the reactants A or B disappear or the rate at which products C or D appear. Experimentally, the change in concentration of A,B,C, and D with time can be measured. The rate of reaction can be expressed as: Rate of disappearance of A= Change in concentration of A=[A] Time required for change t The rate law for the general reaction scheme is given as Rate =k[A][[B]y, where A and B are the molar concentration of reactants, and x and y are orders of the reaction with respect to A and B.k is the rate constant. Adding x and y together will give the overall order of the reaction. One method of determining the order of the reaction is to observe the changes of the reaction as reactant concentrations are varied. The stoichiometry and the order of the reaction have no dependence on each other and usually are different. The rate constant, k, has a definite value for a given reaction and is independent of the concentration. The rate constant only depends on temperature and may be calculated once the rate of the reaction is known. In this experiment, we will study the following chemical reaction: 6I(aq)+BrO3(aq)+6H+(aq)3I2(aq)+Br(aq)+3H2O (Reaction 1 , slow) The rate of reaction 1 depends on the concentration of I,BrO3 and H+in solution. The rate law to be determined is of the form Your goal will be to determine the values of m,n and 0 as well as the specific rate constant k. You will add to the solution a small amount Na2S2O3, which will cause a change in the color of the solution. The blue-black color that will appear in the reaction is due to the presence of stareh-iodine complex that is formed from iodine. The color therefore will not appear until a detectable amount of 12 is formed according to reaction 1 . The thiosulfate (S2O32) that is added to the solution reacts extremely rapidly with iodine as follows: I2(aq)+2S2O32(aq)2r2(aq)+S4O62(aq) (Reaction 2, fist) Consequently, until the same amount of S2O32, that is added, is all consumed, there will not be a sufficient amount of I2 in the solution to yield the blue-black color. The reaction of starch with I2 results in a convenient "clock" to measure the rate at which reaction 1 proceeds. The reaction rate is determined by the time required for a color change to occur. The rates of the reaction will depend on the concentration of the reactants and is proportional to 1/ time. Therefore in this experiment, 1/ time values will be used as substitute for the rate of reaction. Reacting solution are prepared from stock solutions such that the concentration of one component is changed while maintaining all other concentrations constant. This provides a fairly simple way of determining the reaction orders for the different reactants. Procedure Using two 250mL Erlenmeyer flasks, prepare solutions following the table given below. Record room temperature. 1. To begin the reaction, pour the contents of flask 1 into flask 2 and begin timing the reaction as soon as the reagents are poured together. 2. Swirl the solution continually and gently to mix. 3. Record the time when the solution turns blue to the nearest second. 4. Repeat steps 1 through 3 for all trials. Table1. Solution Volumes (mL) Table2. Solution Concentrations Table3. Dependence of reaction rate on concentration Calculations 1. Calculate the exact concentrations of KI,Na2S2O3,KBrO3 and HCl. 2. Using the experimental data collected, evaluate the exponents of the rate law expression. 3. Write out the complete rate law expression for reaction 1 . 4. What is the overall order of the reaction? 5. Calculate the rate constant, k, for each trial by substituting the concentration of r, BrO3, and H+and the values of m,n, and o into rate law expression. 6. Calculate the average rate constant. (Show proper units for the rate constant) 7. If you want to double the rate of the iodine clock reaction, what experimental changes must be made
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


