Question: Can someone help me to answer this correctly, please, and explain it. Please answer these 2 short questions, I don't have more money to buy
Can someone help me to answer this correctly, please, and explain it. Please answer these 2 short questions, I don't have more money to buy more questions. These 2 questions are different.
1. a.
For 1.a. the answers are below

1.b.

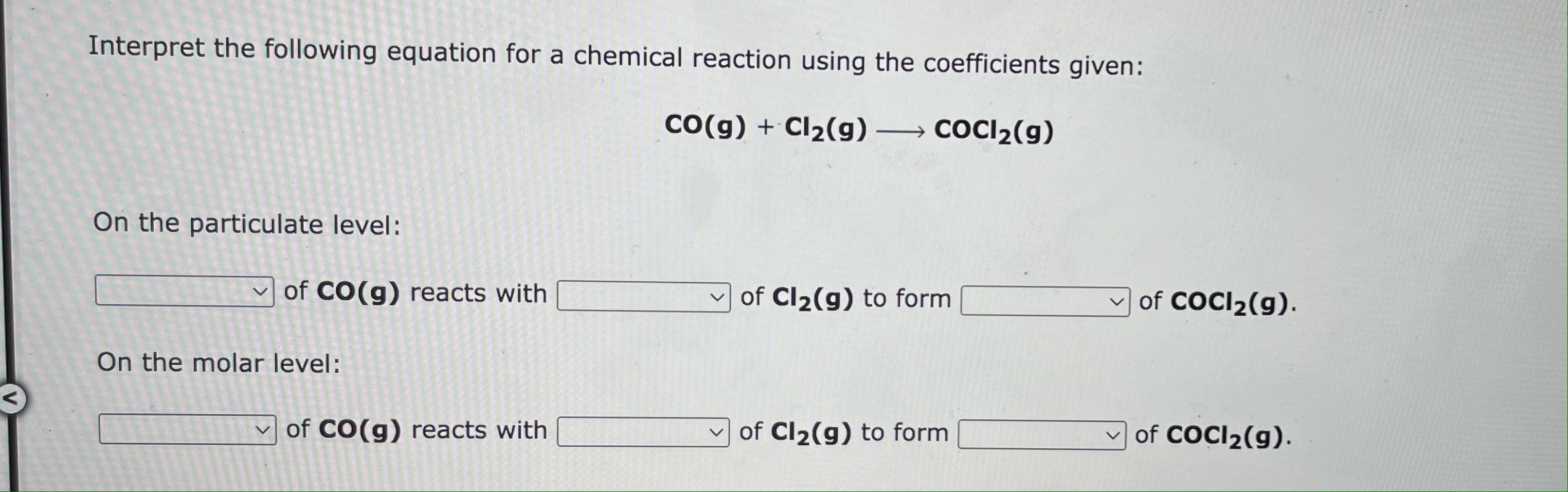
Interpret the following equation for a chemical reaction using the coefficients given: CO(g)+Cl2(g)COCl2(g) On the particulate level: of CO(g) reacts with of Cl2(g) to form of CoCl2(g). On the molar level: of CO(g) reacts with of Cl2(g) to form of CoCl2(g). one molecule two molecules one mole two moles The balanced chemical equation for the reaction between hydrochloric acid and iron(III) oxide is: 6HCl(aq)+Fe2O3(s)3H2O(I)+2FeCl3(aq) We can interpret this to mean: 1 mole of iron(III) oxide and mole(s) of hydrochloric acid React to produce: mole(s) of water and mole(s) of iron(III) chloride
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


