Question: Can someone help me to answer this correctly, please, and explain it. Please answer these 2 questions, I don't have more money to buy more
Can someone help me to answer this correctly, please, and explain it. Please answer these 2 questions, I don't have more money to buy more questions. These 2 questions are different.
1. a.

1. b.
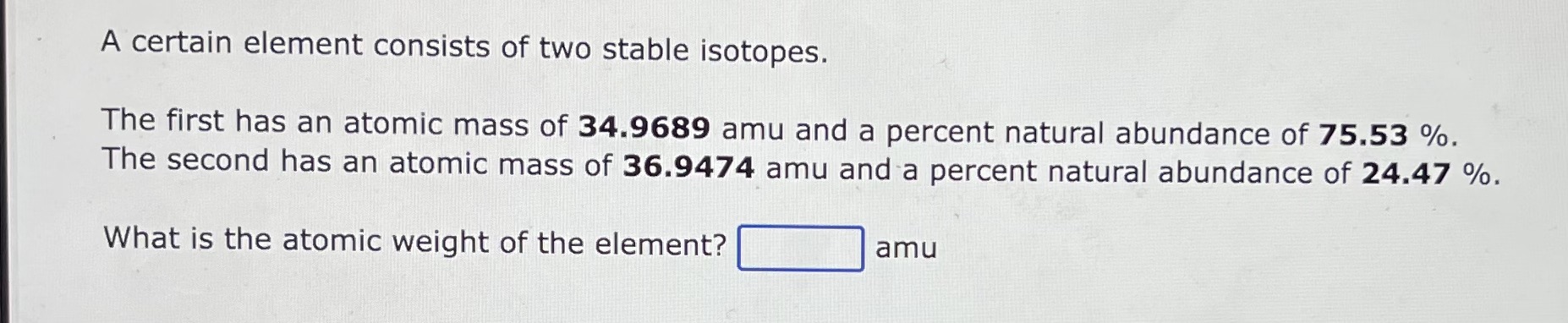
A certain element consists of two stable isotopes. The first has a mass of 35.0 amu and a percent natural abundance of 75.5%. The second has a mass of 36.9 amu and a percent natural abundance of 24.5%. What is the atomic weight of the element? amu A certain element consists of two stable isotopes. The first has an atomic mass of 34.9689 amu and a percent natural abundance of 75.53%. The second has an atomic mass of 36.9474 amu and a percent natural abundance of 24.47%. What is the atomic weight of the element? amu
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


