Question: Can someone help me to answer this correctly, please, and explain it. Please answer these 3 short questions, I don't have more money to buy
Can someone help me to answer this correctly, please, and explain it. Please answer these 3 short questions, I don't have more money to buy more questions. These 3 questions are different.
1. a.

1. b.

1. c.
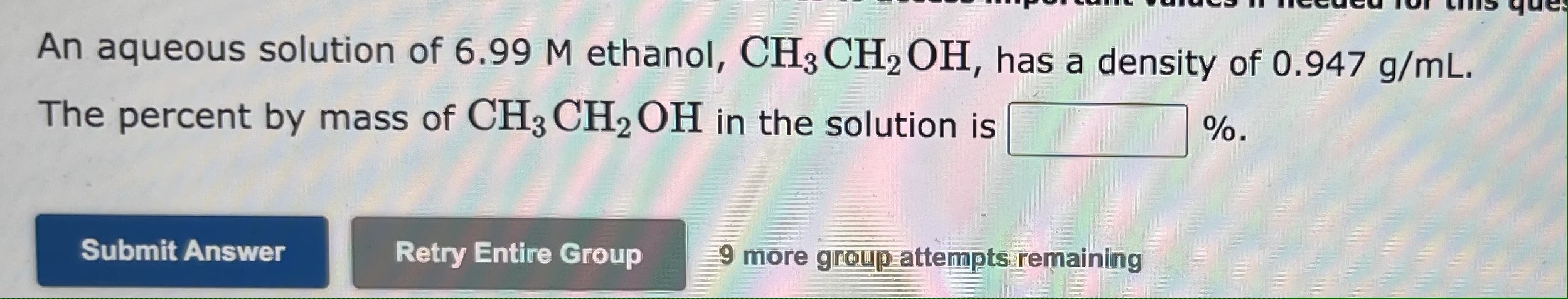
An aqueous solution is 34.0% by mass ethanol, CH3CH2OH, and has a density of 0.947g/mL. The mole fraction of ethanol in the solution is The mole fraction of calcium chloride, CaCl2, in an aqueous solution is 0.0271. The percent by mass of calcium chloride in the solution is % An aqueous solution of 6.99M ethanol, CH3CH2OH, has a density of 0.947g/mL. The percent by mass of CH3CH2OH in the solution is %. 9 more group attempts remaining
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


