Question: Can someone help me to answer this correctly, please, and explain it. Please answer these 2 short questions, I don't have more money to buy
Can someone help me to answer this correctly, please, and explain it. Please answer these 2 short questions, I don't have more money to buy more questions. These 2 questions are different.
1. a.
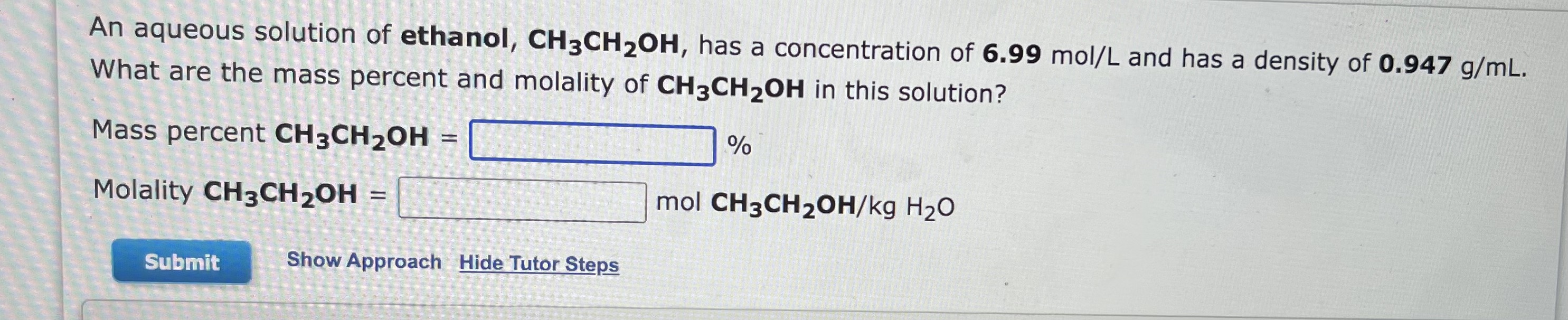
1. b.
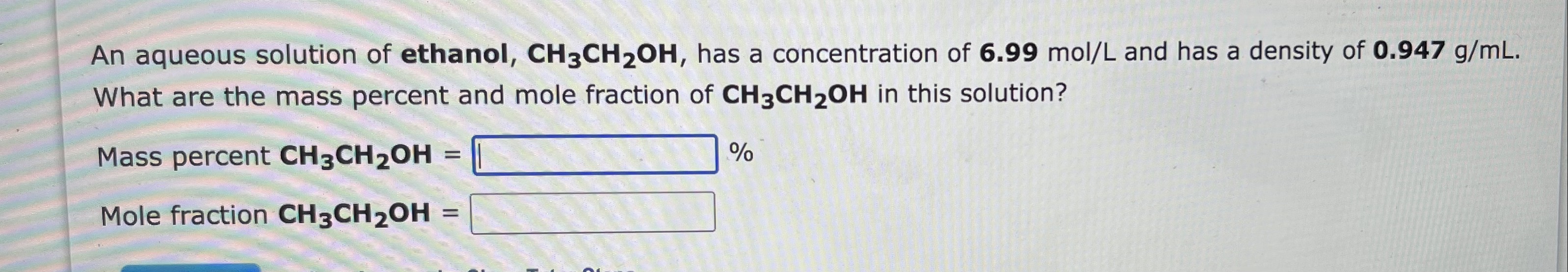
An aqueous solution of ethanol, CH3CH2OH, has a concentration of 6.99mol/L and has a density of 0.947g/mL. What are the mass percent and molality of CH3CH2OH in this solution? Mass percent CH3CH2OH= % Molality CH3CH2OH= molCH3CH2OH/kgHH2O Show Approach Hide Tutor Steps An aqueous solution of ethanol, CH3CH2OH, has a concentration of 6.99mol/L and has a density of 0.947g/mL. What are the mass percent and mole fraction of CH3CH2OH in this solution? Mass percent CH3CH2OH= % Mole fraction CH3CH2OH=
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


