Question: Can someone help me to answer this correctly, please, and explain it. Please answer these 2 short questions, I don't have more money to buy
Can someone help me to answer this correctly, please, and explain it. Please answer these 2 short questions, I don't have more money to buy more questions. These 2 questions are different.
1. a.
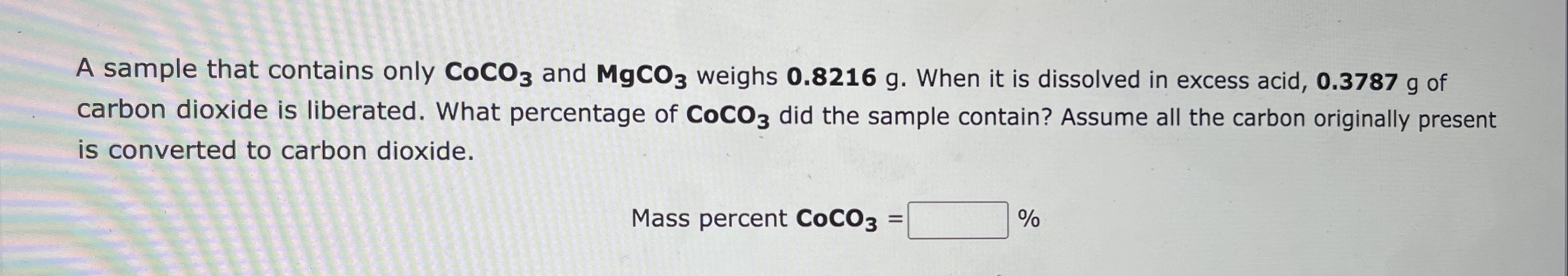
1. b.

A sample that contains only CoCO3 and MgCO3 weighs 0.8216g. When it is dissolved in excess acid, 0.3787g of carbon dioxide is liberated. What percentage of CoCO3 did the sample contain? Assume all the carbon originally present is converted to carbon dioxide. MasspercentCoCO3=% Consider this reaction, which occurs in the atmosphere and contributes to photochemical smog: Br2(g)+Cl2(g)2BrCl(g) If there is 18.39gBr2 and excess Cl2 present, the reaction yields 18.8gBrCl. Calculate the percent yield for the reaction. %
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


