Question: Can someone help me to answer this correctly, please, and explain it. Please answer these 3 short questions, I don't have more money to buy
Can someone help me to answer this correctly, please, and explain it. Please answer these 3 short questions, I don't have more money to buy more questions. These 3 questions are different.
1. a.
1. b.

1. c.

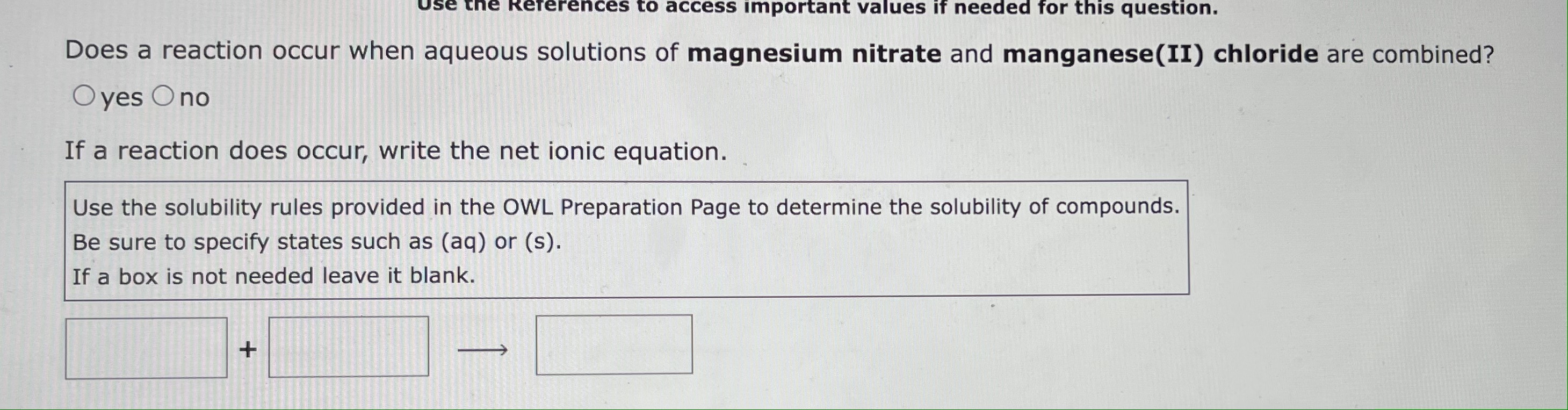
Does a reaction occur when aqueous solutions of magnesium nitrate and manganese(II) chloride are combined? yes no If a reaction does occur, write the net ionic equation. Use the solubility rules provided in the OWL Preparation Page to determine the solubility of compounds. Be sure to specify states such as (aq) or (s). If a box is not needed leave it blank. Write a balanced equation for the double-replacement precipitation reaction described, using the smallest possible integer coefficients. A precipitate forms when aqueous solutions of nickel(II) iodide and sodium hydroxide are combined. Be sure to include states such as (s) or (aq). Consider the reaction when aqueous solutions of aluminum iodide and potassium phosphate are combined. The net ionic equation for this reaction is: (Be sure to specify states such as (aq) or (s). If a box is not needed leave it blank. If no reaction occurs leave all boxes blank and click on "submit".)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


