Question: Can someone help me to answer this correctly, please, and explain it. Please answer these 2 short questions, I don't have more money to buy
Can someone help me to answer this correctly, please, and explain it. Please answer these 2 short questions, I don't have more money to buy more questions. These 2 questions are different.
1. a.
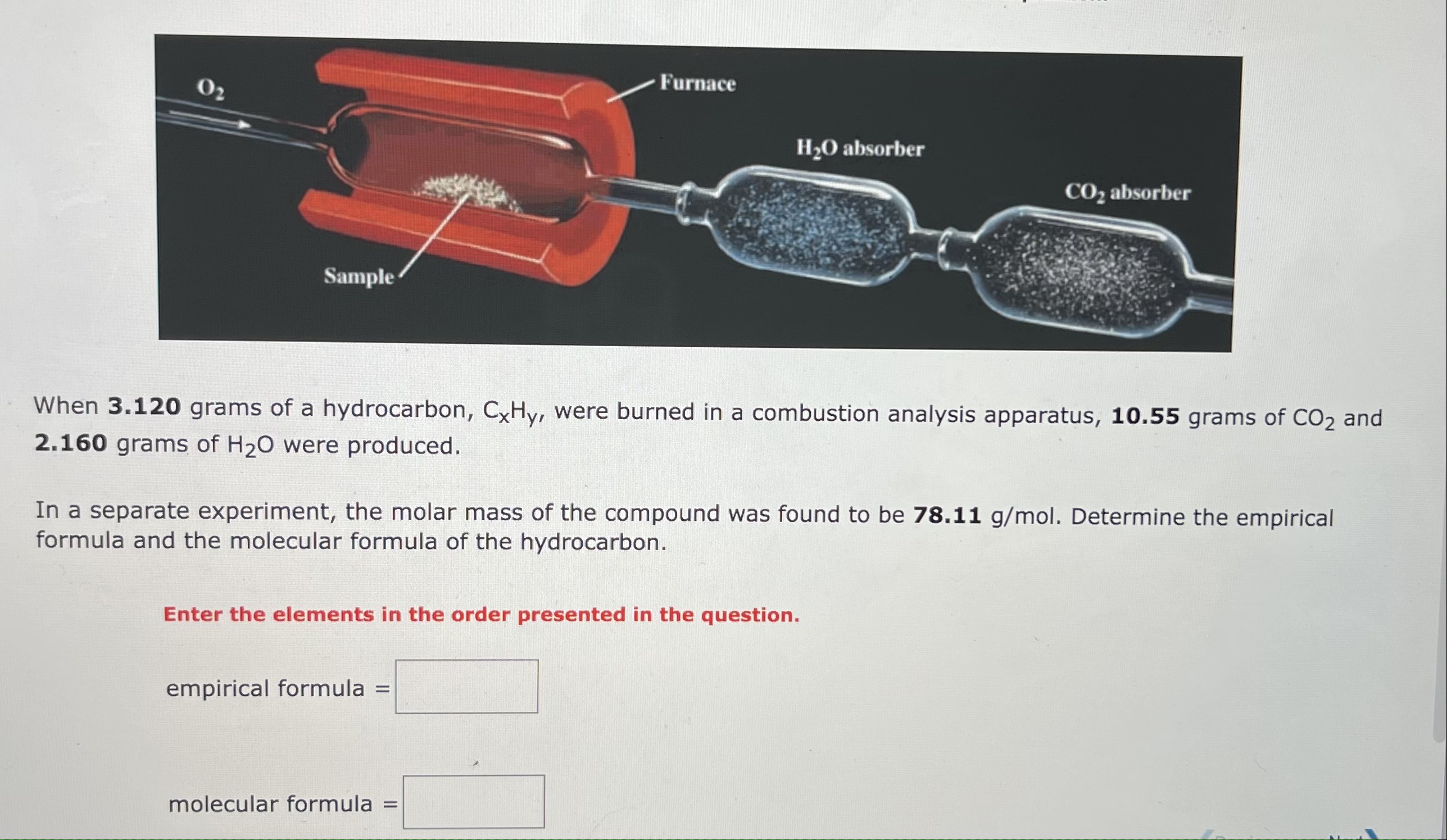
1. b.
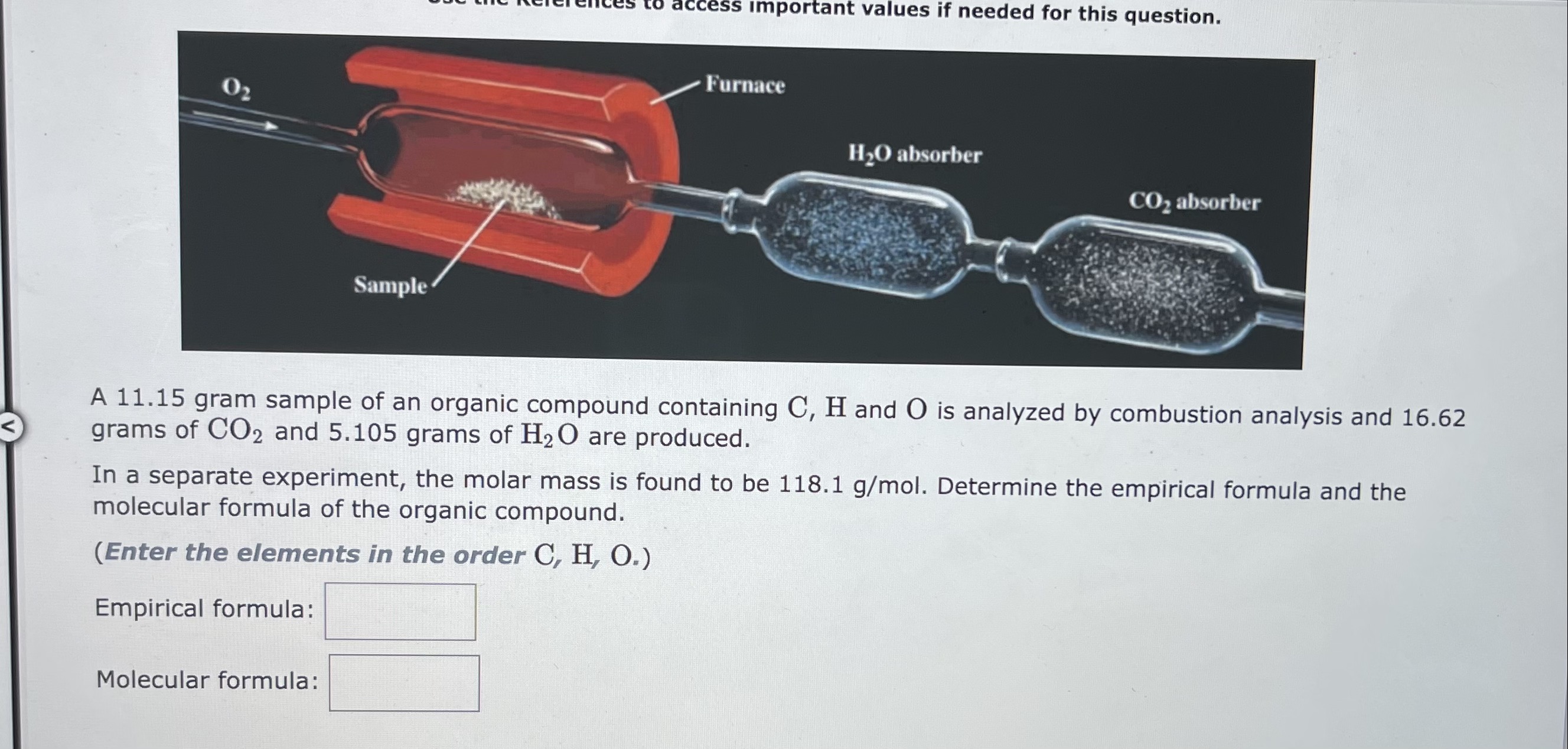
When 3.120 grams of a hydrocarbon, CxHy, were burned in a combustion analysis apparatus, 10.55grams of CO2 and 2.160 grams of H2O were produced. In a separate experiment, the molar mass of the compound was found to be 78.11g/mol. Determine the empirical formula and the molecular formula of the hydrocarbon. Enter the elements in the order presented in the question. empirical formula = molecular formula = A 11.15 gram sample of an organic compound containing C,H and O is analyzed by combustion analysis and 16.62 grams of CO2 and 5.105 grams of H2O are produced. In a separate experiment, the molar mass is found to be 118.1g/mol. Determine the empirical formula and the molecular formula of the organic compound. (Enter the elements in the order C,H,O.) Empirical formula: Molecular formula
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


