Question: Can someone help me to answer this correctly, please, and explain it. Please answer these 2 short questions, I don't have more money to buy
Can someone help me to answer this correctly, please, and explain it. Please answer these 2 short questions, I don't have more money to buy more questions. These 2 questions are different.
1. a.

1. b.
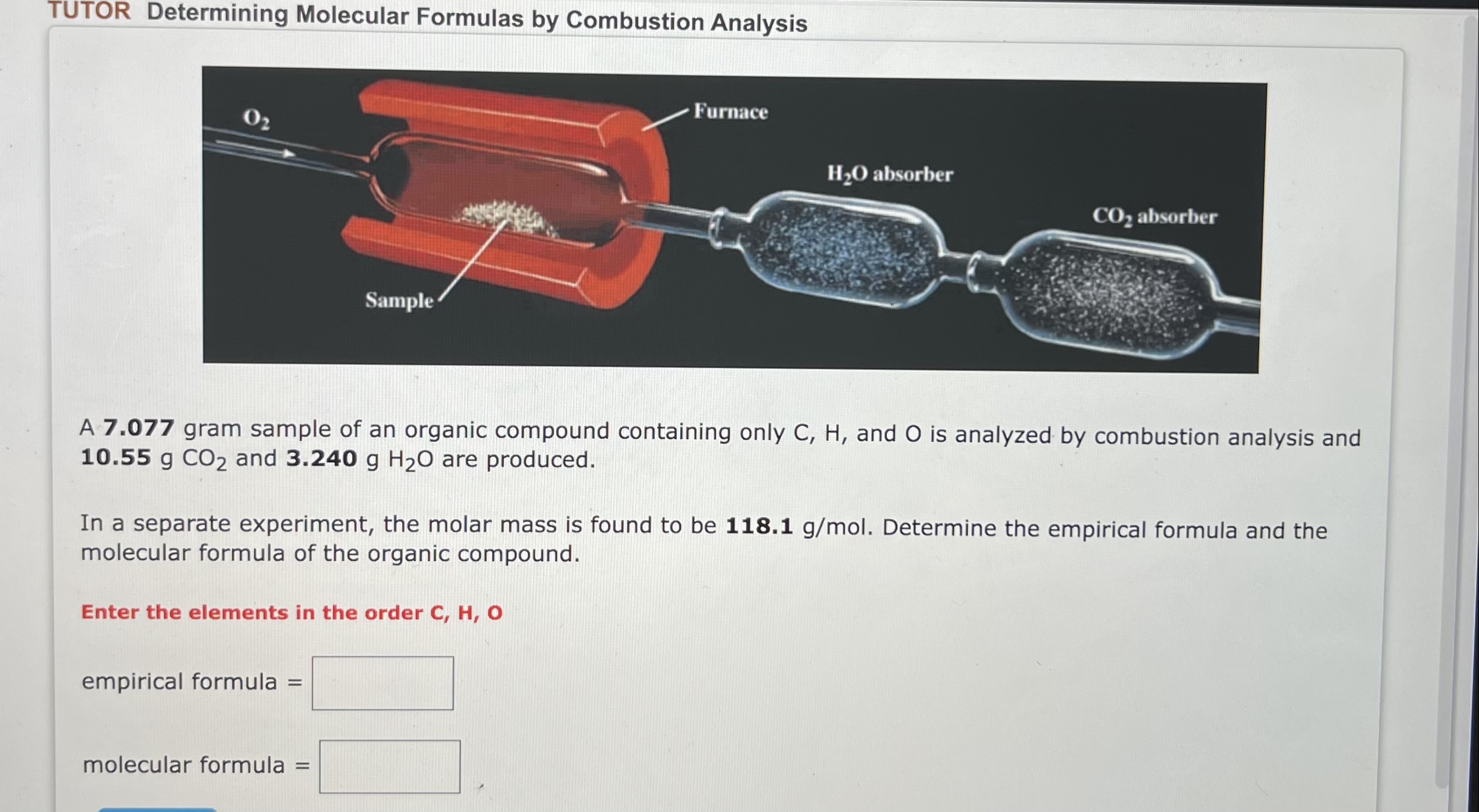
For the following reaction, 23.5 grams of nitrogen monoxide are allowed to react with 1.37 grams of hydrogen gas. nitrogenmonoxide(g)+hydrogen(g)nitrogen(g)+water(l) What is the maximum amount of nitrogen gas that can be formed? Mass=g What is the formula for the limiting reagent? What amount of the excess reagent remains after the reaction is complete? Mass = A 7.077 gram sample of an organic compound containing only C,H, and O is analyzed by combustion analysis and 10.55gCO2 and 3.240gH2O are produced. In a separate experiment, the molar mass is found to be 118.1g/mol. Determine the empirical formula and the molecular formula of the organic compound. Enter the elements in the order C,H,O empirical formula = molecular formula =
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


