Question: Can someone help me understand how do I do this? Given the reaction CH3COCH3(l)+4O2(g)3CO2(g)+3H2O(g)H=1657.6kJ/mol Determine the Hf for CH3COCH3(l)
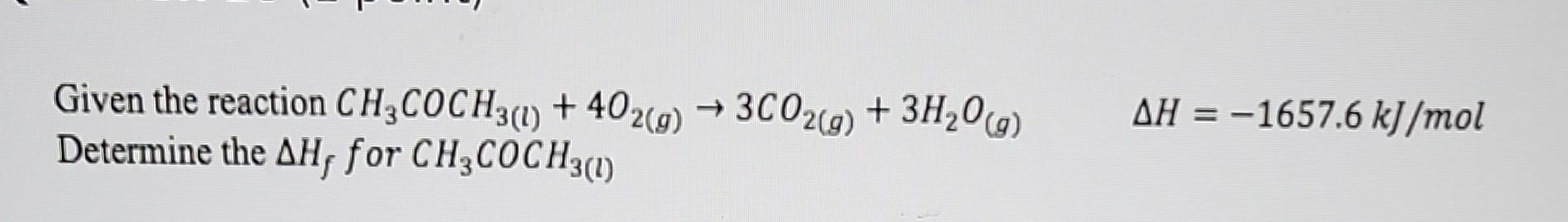
Can someone help me understand how do I do this?
Given the reaction CH3COCH3(l)+4O2(g)3CO2(g)+3H2O(g)H=1657.6kJ/mol Determine the Hf for CH3COCH3(l)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


