Question: Can someone help me understand this problem better and show all hand calculations pls. 6. Purge Analysis: For this purge system, the amount of inert
Can someone help me understand this problem better and show all hand calculations pls.
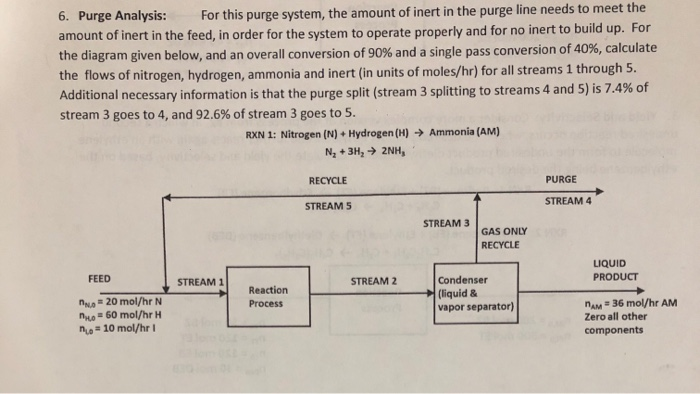
6. Purge Analysis: For this purge system, the amount of inert in the purge line needs to meet the amount of inert in the feed, in order for the system to operate properly and for no inert to build up. For the diagram given below, and an overall conversion of 90% and a single pass conversion of 40%, calculate the flows of nitrogen, hydrogen, ammonia and inert (in units of moles/hr) for all streams 1 through 5. Additional necessary information is that the purge split (stream 3 splitting to streams 4 and 5) is 7.4% of stream 3 goes to 4, and 92.6% of stream 3 goes to 5. RXN 1: Nitrogen (N) + Hydrogen (H) Ammonia (AM) N2 + 3H2 2NH, RECYCLE PURGE STREAM 4 STREAM 5 STREAM 3 GAS ONLY RECYCLE LIQUID PRODUCT FEED STREAM 1 STREAM 2 Reaction Process Condenser (liquid & vapor separator) ANO = 20 mol/hr N no 50 mol/hr H no = 10 mol/hr nam = 36 mol/hr AM Zero all other components
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


