Question: Can someone help me with part 2 and part 3. I have included part 1 because it connects with part 2. The + means that
Can someone help me with part 2 and part 3. I have included part 1 because it connects with part 2. The + means that it showed a reaction while the - means there was no reaction
Part 1 Provided Below
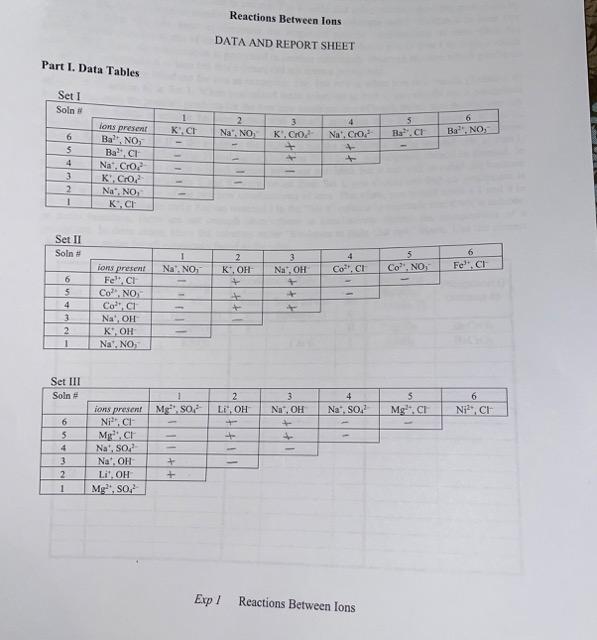
Part 2 Provided Below
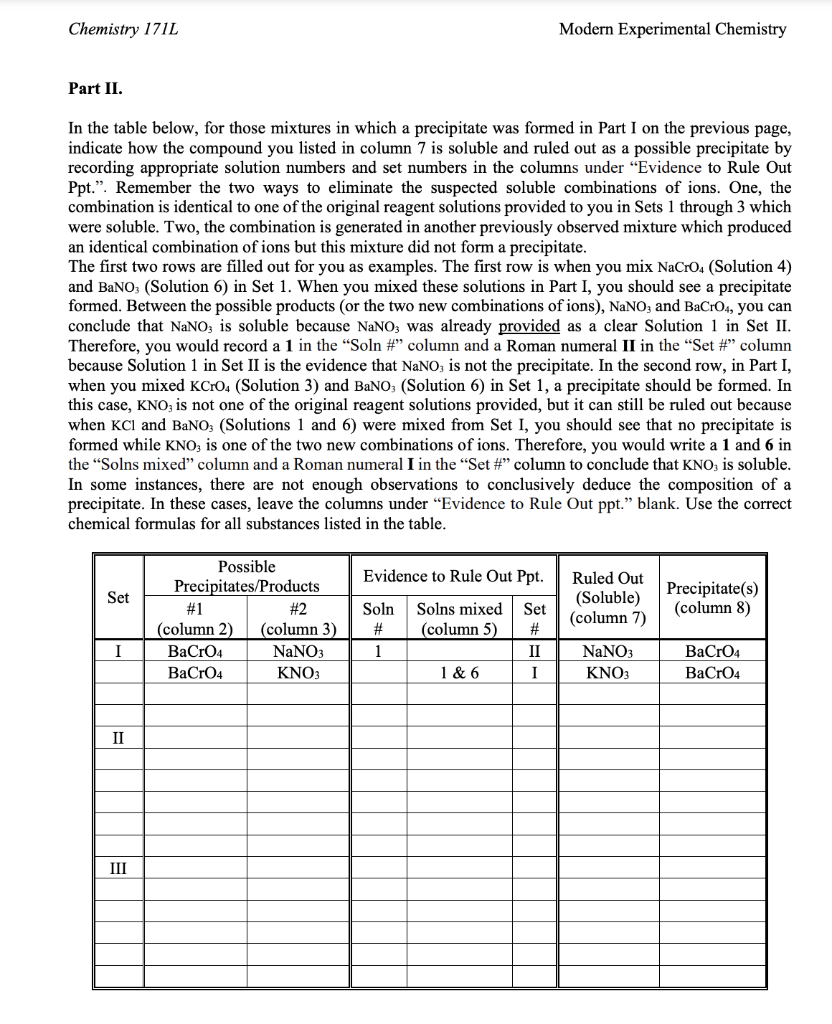
Part 3 Provided Below

Reactions Between Ions DATA AND REPORT SHEET Part I. Data Tables Set II Exp I Reactions Between Ions Chemistry 171L Modern Experimental Chemistry Part II. In the table below, for those mixtures in which a precipitate was formed in Part I on the previous page, indicate how the compound you listed in column 7 is soluble and ruled out as a possible precipitate by recording appropriate solution numbers and set numbers in the columns under "Evidence to Rule Out Ppt.". Remember the two ways to eliminate the suspected soluble combinations of ions. One, the combination is identical to one of the original reagent solutions provided to you in Sets 1 through 3 which were soluble. Two, the combination is generated in another previously observed mixture which produced an identical combination of ions but this mixture did not form a precipitate. The first two rows are filled out for you as examples. The first row is when you mixNaCrO4 (Solution 4) and BaNO3 (Solution 6) in Set 1. When you mixed these solutions in Part I, you should see a precipitate formed. Between the possible products (or the two new combinations of ions), NaNO3 and BaCrO4, you can conclude that NaNO3 is soluble because NaNO3 was already provided as a clear Solution 1 in Set II. Therefore, you would record a 1 in the "Soln \#" column and a Roman numeral II in the "Set \#" column because Solution 1 in Set II is the evidence that NaNO3 is not the precipitate. In the second row, in Part I, when you mixed KCrO4 (Solution 3) and BaNO3 (Solution 6) in Set 1, a precipitate should be formed. In this case, KNO3 is not one of the original reagent solutions provided, but it can still be ruled out because when KCl and BaNO3 (Solutions 1 and 6) were mixed from Set I, you should see that no precipitate is formed while KNO3 is one of the two new combinations of ions. Therefore, you would write a 1 and 6 in the "Solns mixed" column and a Roman numeral I in the "Set \#" column to conclude that KNO3 is soluble. In some instances, there are not enough observations to conclusively deduce the composition of a precipitate. In these cases, leave the columns under "Evidence to Rule Out ppt." blank. Use the correct chemical formulas for all substances listed in the table. Chemistry 171L Modern Experimental Chemistry Part III. In Set III, LiCl could not be ruled out as a possible precipitate. Suggest an additional experiment and explain how one could definitively assess the solubility of LiCl using any two reagent solutions from Set I to III you were provided with. Provide a specific combination of two reagent solutions and do not use the solubility rule in your explanation. (Hint: LiCl should be the product of the additional experiment, not a reagent). Questions: 1. Write balanced equations showing the dissociation of the following compounds in an aqueous solution: a) NaBr b) CaCl2 c) Na3PO4 d) Na2CO3 e) Mg(NO3)2 f) Al2(SO4)3 g) Fe(NO3)3 h) (NH4)2SO4 2. a) Write a balanced complete ionic equation with state symbols representing the mixing of an aqueous solution of iron (III) chloride with an aqueous solution of sodium hydroxide, keeping in mind that a precipitate forms when these two solutions are mixed. b) Write the balanced net ionic equation
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


