Question: can someone help me with the coding for this on MATLAB please. Composition of Air It is often useful to think of air as a
can someone help me with the coding for this on MATLAB please.
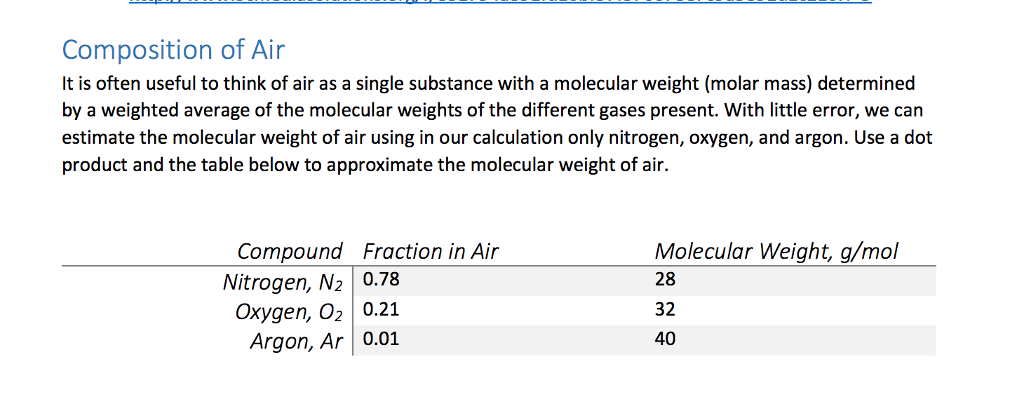
Composition of Air It is often useful to think of air as a single substance with a molecular weight (molar mass) determined by a weighted average of the molecular weights of the different gases present. With little error, we can estimate the molecular weight of air using in our calculation only nitrogen, oxygen, and argon. Use a dot product and the table below to approximate the molecular weight of air. Molecular Weight, g/mol 28 32 40 Compound Fraction in Air Nitrogen, N2 0.78 Oxygen, O2 0.21 Argon, Ar 0.01 Composition of Air It is often useful to think of air as a single substance with a molecular weight (molar mass) determined by a weighted average of the molecular weights of the different gases present. With little error, we can estimate the molecular weight of air using in our calculation only nitrogen, oxygen, and argon. Use a dot product and the table below to approximate the molecular weight of air. Molecular Weight, g/mol 28 32 40 Compound Fraction in Air Nitrogen, N2 0.78 Oxygen, O2 0.21 Argon, Ar 0.01
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


