Question: can someone help me with these question? 2. 3. Resonance to the Rescue (again) /1 points Once again, a single Lewis structure for a molecule
can someone help me with these question?

2. 
3. 
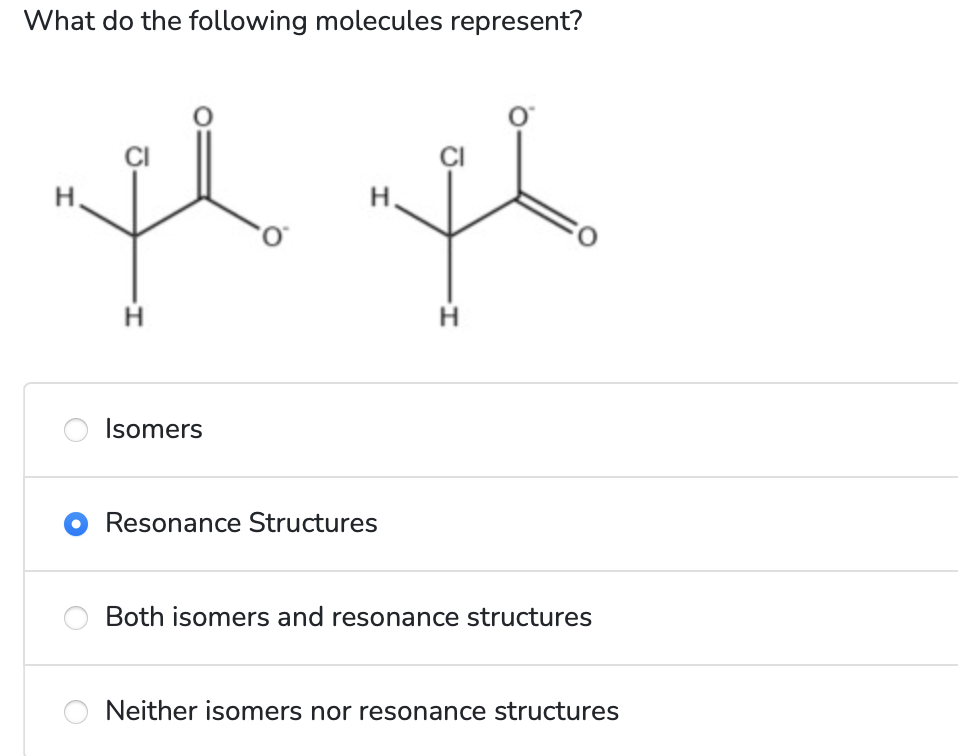
Resonance to the Rescue (again) /1 points Once again, a single Lewis structure for a molecule failed to predict its actual structure. In your notebook, try drawing a resonance structure for acetamide that could explain the actual molecular structure for the nitrogen atom. Enter your resonance structure in the space below: Hint \#1: Your resonance structure will have to break the general bonding rules for nitrogen and oxygen. Hint \#2: When drawing resonance structures, all of the atoms should be kept in the exact same position (you don't want to end up with an isomer). The only thing that will change is the distribution of the bonds and lone pairs. Hint \#3: Some of the atoms in your resonance structure will have non-zero formal charges. Remember to include these formal charges in your drawing. Note that you are drawing a single resonance structure for this question. It should be a different resonance form than the one you drew above. What do the following molecules represent? Isomers Resonance Structures Both isomers and resonance structures Neither isomers nor resonance structures Enter the molecular shape around the central atom(s) for N2O2 linear bent trigonal planar trigonal pyramidal tetrahedral
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


