Question: can someone help with this You have a 15mL sample of acetylcholine (a neurotransmitter) with an unknown concentration and a pH of 7.59. You incubate
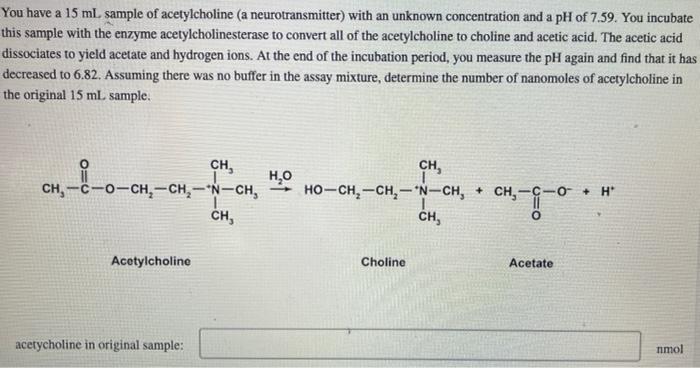
You have a 15mL sample of acetylcholine (a neurotransmitter) with an unknown concentration and a pH of 7.59. You incubate this sample with the enzyme acetylcholinesterase to convert all of the acetylcholine to choline and acetic acid. The acetic acid dissociates to yield acetate and hydrogen ions. At the end of the incubation period, you measure the pH again and find that it has decreased to 6.82. Assuming there was no buffer in the assay mixture, determine the number of nanomoles of acetylcholine in the original 15mL sample. Acotylcholine Choline Acetate acetycholine in original sample: nmol
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


