Question: Can someone please do the calculations for the first row (row 19), so i can fill the rest of the tables? I got no clue
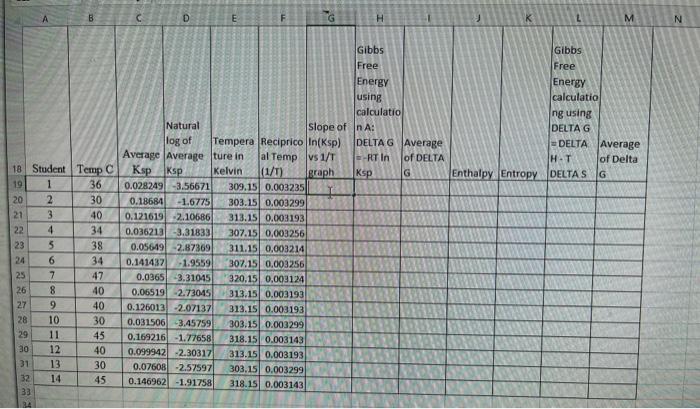
B D E F H K L M N Gibbs Free Energy calculatio ng using DELTAG DELTA Average HT of Delta Enthalpy Entropy DELTAS G WAWN- mm 18 Student Temp C 19 1 36 20 2 30 21 3 40 22 34 23 5 38 24 6 34 25 7 47 26 8 40 27 9 40 28 10 30 29 11 45 30 12 40 31 13 30 32 14 45 33 Gibbs Free Energy using calculatio Natural Slope of n A: log of Tempera Reciprico In(Ksp) DELTAG Average Average Average ture in al Temp vs 1/T RTIN of DELTA Ksp Ksp Kelvin (1/1) graph Ksp G 0.028249 -3.56671 309.15 0.003235 0.18684 -1.6775 303.15 0.003299 0.121619 2.10686 313.15 0.003193 0.036213 -3.31833 307.15 0.003256 0.056491 2.87369 311.15 0.003214 0.141437 -1.9559 307.15 0.003256 0.0365 -3.31045 320.15 0,003124 0.06519 -2.73045 313.15 0.003193 0.126013 -2.07137 313.15 0.003193 0.031506 3.45759 303.15 0.003299 0.169216 -1.77658 318.15 0.003143 0.099942 -2.30317 313.15 0.003193 0.07608 -2.57597 303.15 0.003299 0.146962 -1.91758 318.15 0.003143 RE B D E F H K L M N Gibbs Free Energy calculatio ng using DELTAG DELTA Average HT of Delta Enthalpy Entropy DELTAS G WAWN- mm 18 Student Temp C 19 1 36 20 2 30 21 3 40 22 34 23 5 38 24 6 34 25 7 47 26 8 40 27 9 40 28 10 30 29 11 45 30 12 40 31 13 30 32 14 45 33 Gibbs Free Energy using calculatio Natural Slope of n A: log of Tempera Reciprico In(Ksp) DELTAG Average Average Average ture in al Temp vs 1/T RTIN of DELTA Ksp Ksp Kelvin (1/1) graph Ksp G 0.028249 -3.56671 309.15 0.003235 0.18684 -1.6775 303.15 0.003299 0.121619 2.10686 313.15 0.003193 0.036213 -3.31833 307.15 0.003256 0.056491 2.87369 311.15 0.003214 0.141437 -1.9559 307.15 0.003256 0.0365 -3.31045 320.15 0,003124 0.06519 -2.73045 313.15 0.003193 0.126013 -2.07137 313.15 0.003193 0.031506 3.45759 303.15 0.003299 0.169216 -1.77658 318.15 0.003143 0.099942 -2.30317 313.15 0.003193 0.07608 -2.57597 303.15 0.003299 0.146962 -1.91758 318.15 0.003143 RE
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


