Question: Can someone please explain how he got 0.8 kg? Like what is the step by step algebra to get that answer? Ethylene glycol is used
Can someone please explain how he got 0.8 kg? Like what is the step by step algebra to get that answer?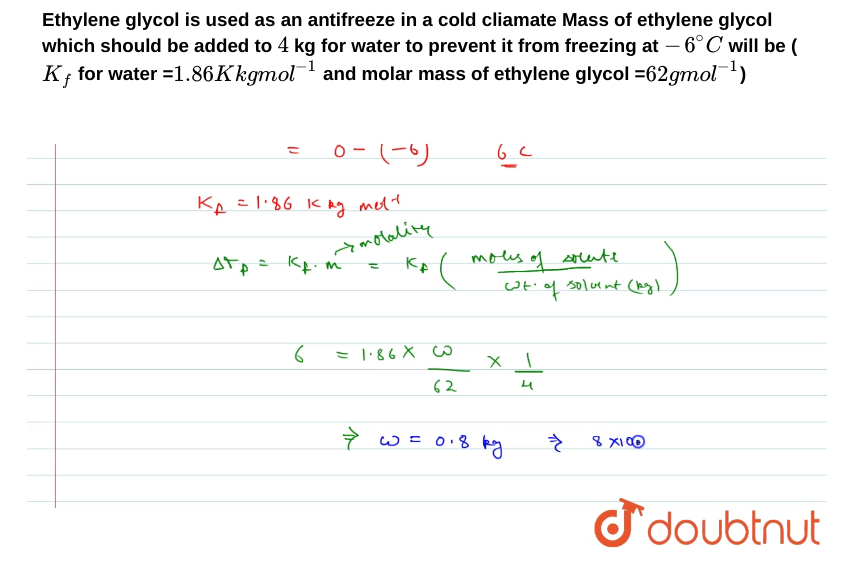
Ethylene glycol is used as an antifreeze in a cold cliamate Mass of ethylene glycol which should be added to 4kg for water to prevent it from freezing at 6C will be ( Kf for water =1.86Kkgmol1 and molar mass of ethylene glycol =62gmol1 ) =0(6)6cKf=1.86kkgmolltP=Kforolality=kf(wt.ofsolunt(kg)molisofsolente)=1.866241=0.8kg8100
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


