Question: Can someone please explain how to do these problems? What expression is equal to Kb for HPO42 ? H3PO4(aq)+H2O(l)H2PO4(aq)+H3O+(aq),Ka1H2PO4(aq)+H2O(I)HPO42(aq)+H3O+(aq),Ka2HPO42(aq)+H2O(I)PO43(aq)+H3O+(aq),Ka3 Kb=Ka2Kw Kb=Ka1 Kb=Ka3Kw Kb=Ka1Kw Kb=Ka2 Kb=Ka3
Can someone please explain how to do these problems?
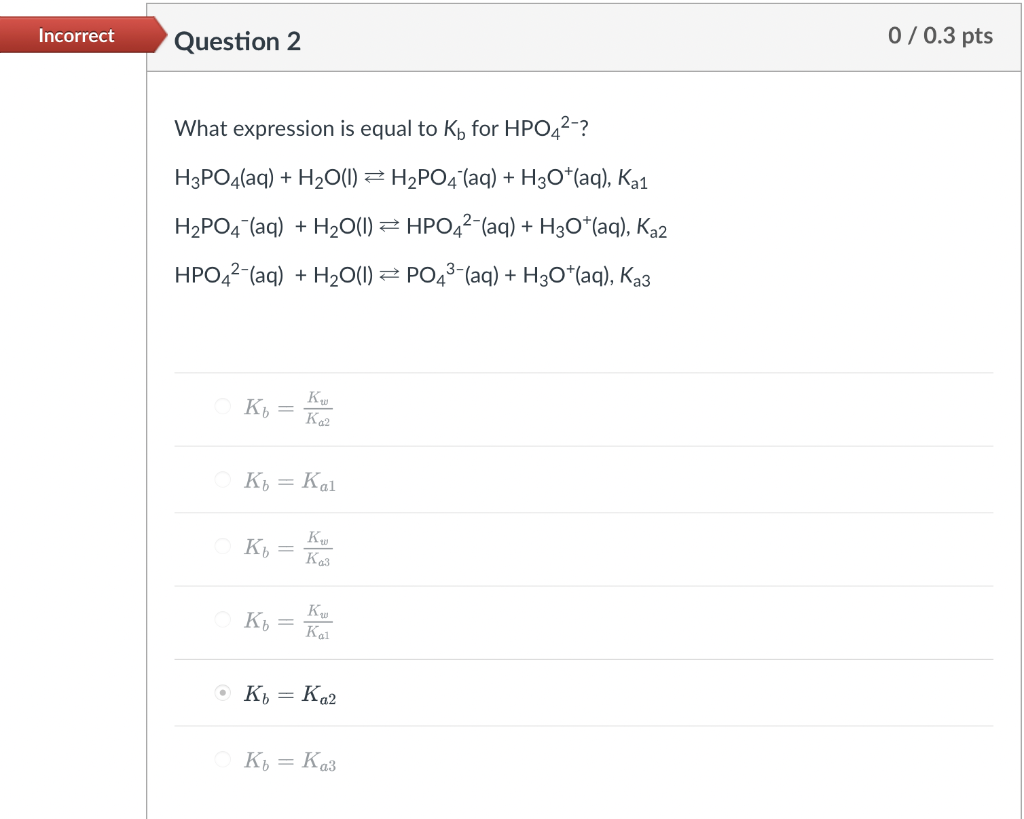
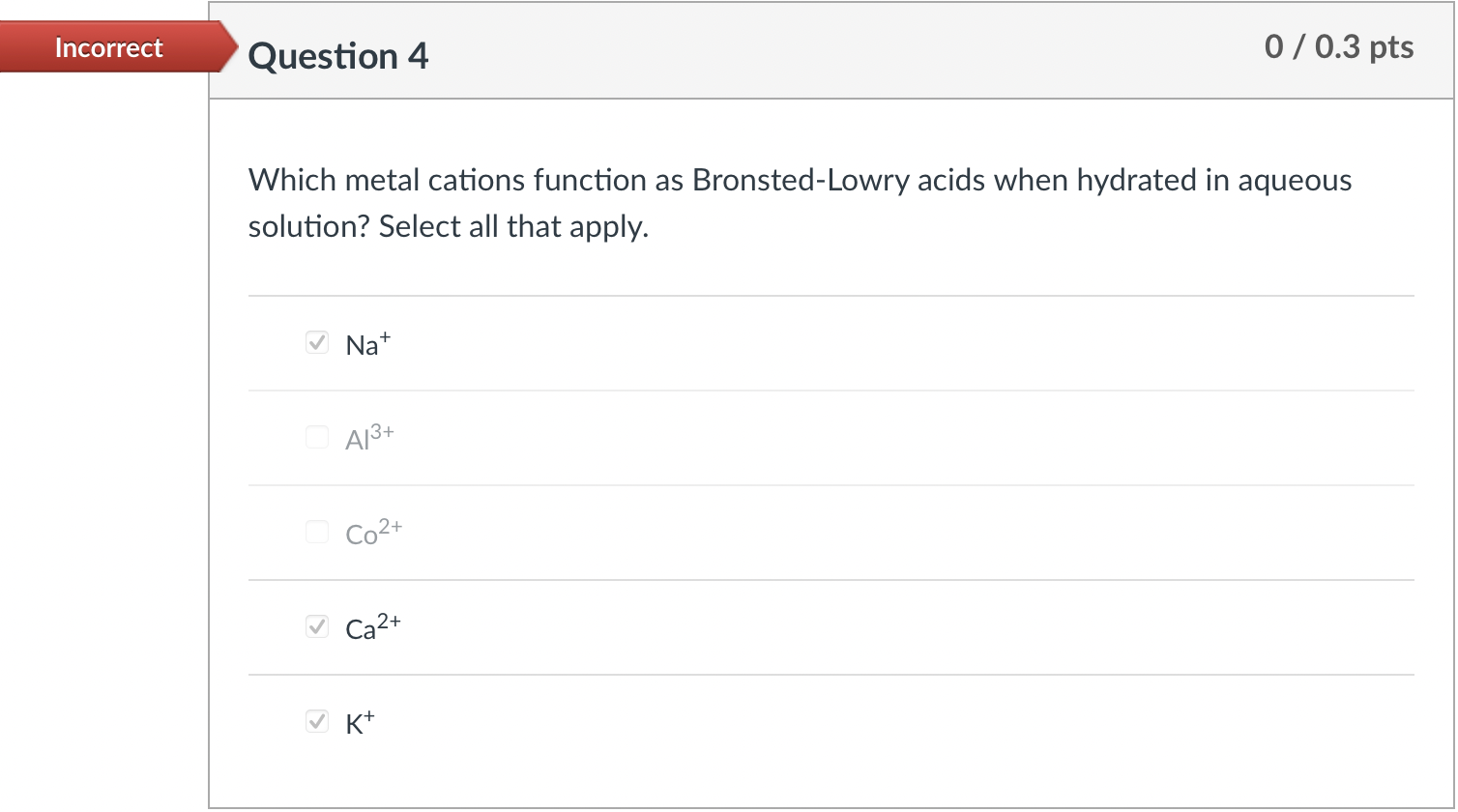
What expression is equal to Kb for HPO42 ? H3PO4(aq)+H2O(l)H2PO4(aq)+H3O+(aq),Ka1H2PO4(aq)+H2O(I)HPO42(aq)+H3O+(aq),Ka2HPO42(aq)+H2O(I)PO43(aq)+H3O+(aq),Ka3 Kb=Ka2Kw Kb=Ka1 Kb=Ka3Kw Kb=Ka1Kw Kb=Ka2 Kb=Ka3 Which metal cations function as Bronsted-Lowry acids when hydrated in aqueous solution? Select all that apply. Na+ Al3+ Co2+ Ca2+ K+
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


