Question: can someone please go over my work and make sure I did it correct before turning it in my answer is this reaction is a
can someone please go over my work and make sure I did it correct before turning it in
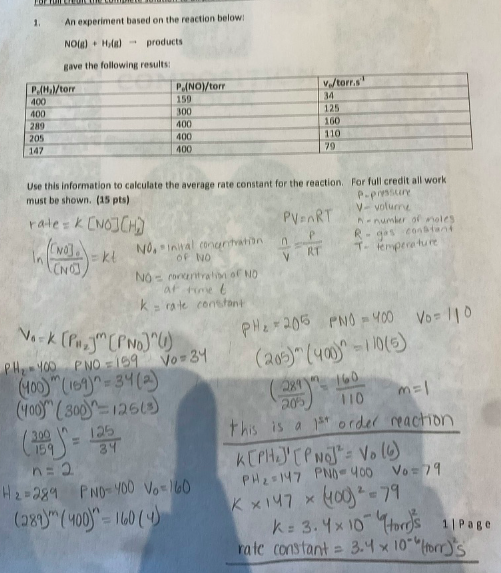
my answer is this reaction is a 1st order reaction and the rate constant is 3.4x10^-6 (torr)^2s
NO(g)+H2(g)products gave the following results: Use this information to calculate the average rate constant for the reaction. For full credit all work must be shown. (15 pts) P= pinsistire rate =K[NO][H3] V= volurine NO(g)+H2(g)products gave the following results: Use this information to calculate the average rate constant for the reaction. For full credit all work must be shown. (15 pts) P= pinsistire rate =K[NO][H3] V= volurine
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


