Question: Can someone please help me with the resonance structures for the two skeletons and also with question #4. Thank you so much :)) 3. Using
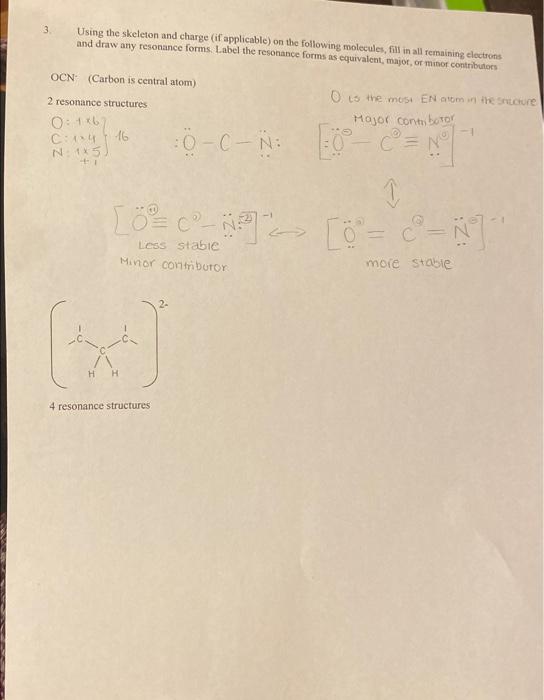
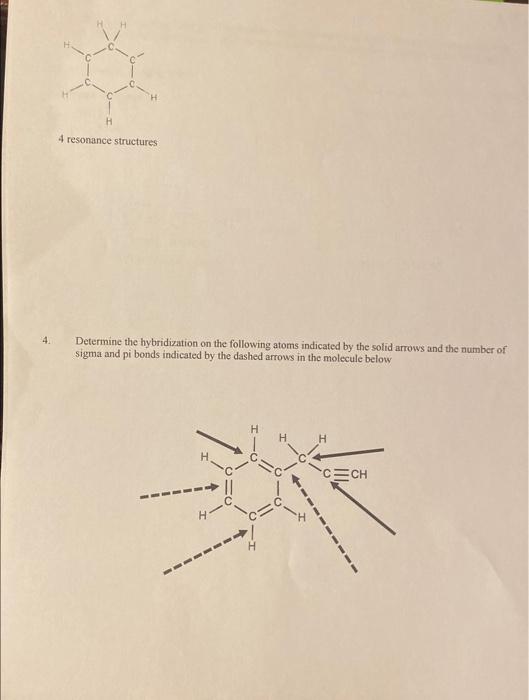
3. Using the skeleton and charge (if applicable) on the following molecules, fill in all remaining electrons and draw any resonance forms. Label the resonance forms as equivalent, major, of minor contributors OCN(Carbon is central atom) 2 resonance structures Q. is the most EN atom in the srete Minor comtributor more stable 4 resonance structures 4 resonance structures 4. Determine the hybridization on the following atoms indicated by the solid arrows and the number of sigma and pi bonds indicated by the dashed arrows in the molecule below
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


