Question: can someone please show me how to do the structures correctly? Resources Give Up? Hint estion 21 of 23 > Finish resonance structure 3 Select
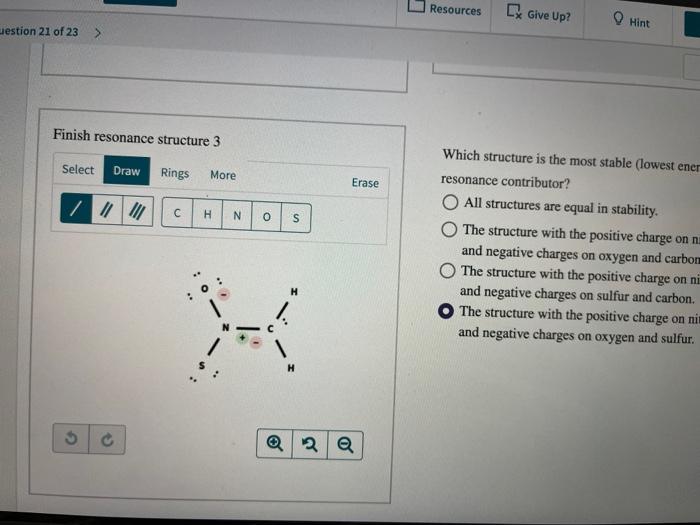
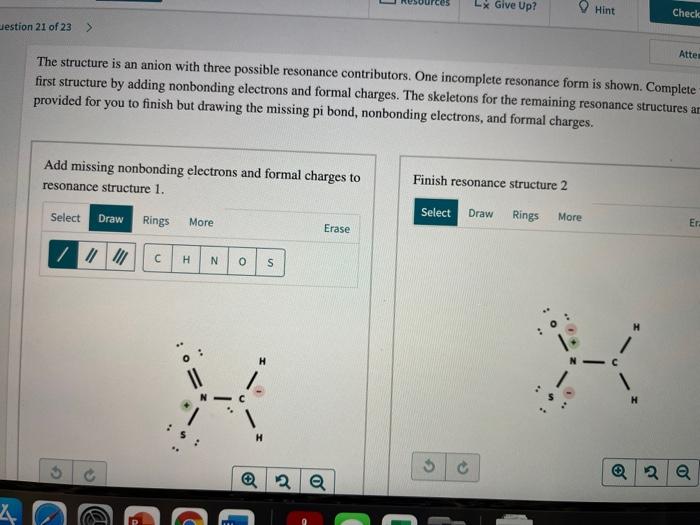
Resources Give Up? Hint estion 21 of 23 > Finish resonance structure 3 Select Draw Rings More Erase III H N O S Which structure is the most stable (lowest ener resonance contributor? O All structures are equal in stability. The structure with the positive charge on na and negative charges on oxygen and carbon The structure with the positive charge on ni and negative charges on sulfur and carbon. The structure with the positive charge on ni and negative charges on oxygen and sulfur. H H 2 a LX Give Up? Hint Check destion 21 of 23 > Atte The structure is an anion with three possible resonance contributors. One incomplete resonance form is shown. Complete first structure by adding nonbonding electrons and formal charges. The skeletons for the remaining resonance structures ar provided for you to finish but drawing the missing pi bond, nonbonding electrons, and formal charges. Add missing nonbonding electrons and formal charges to resonance structure 1. Finish resonance structure 2 Select Select Draw Draw Rings Rings More More Erase Er // II H N o 0 s H . H 5 2 2 o a
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


