Question: can u please help me with these four questions 33. Calculate the molarity (M) of a solution containing 12.0 moles of KNO3 and 5000mL of
can u please help me with these four questions 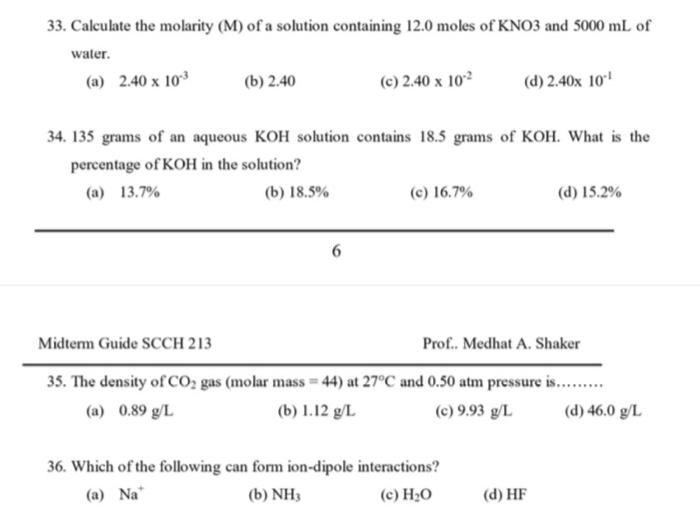

33. Calculate the molarity (M) of a solution containing 12.0 moles of KNO3 and 5000mL of water. (a) 2.40103 (b) 2.40 (c) 2.40102 (d) 2.40101 34. 135 grams of an aqueous KOH solution contains 18.5 grams of KOH. What is the percentage of KOH in the solution? (a) 13.7% (b) 18.5% (c) 16.7% (d) 15.2% Midterm Guide SCCH 213 Prof.. Medhat A. Shaker 35. The density of CO2 gas (molar mass =44 ) at 27C and 0.50 atm pressure is. (a) 0.89g/L (b) 1.12g/L (c) 9.93g/L (d) 46.0g/L 36. Which of the following can form ion-dipole interactions? (a) Na+ (b) NH3 (c) H2O (d) HF
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


