Question: can u show the working step by step Exercise 08 - Effect of concentration changes on equilibrium yields (20 marks) The equation shows a reaction
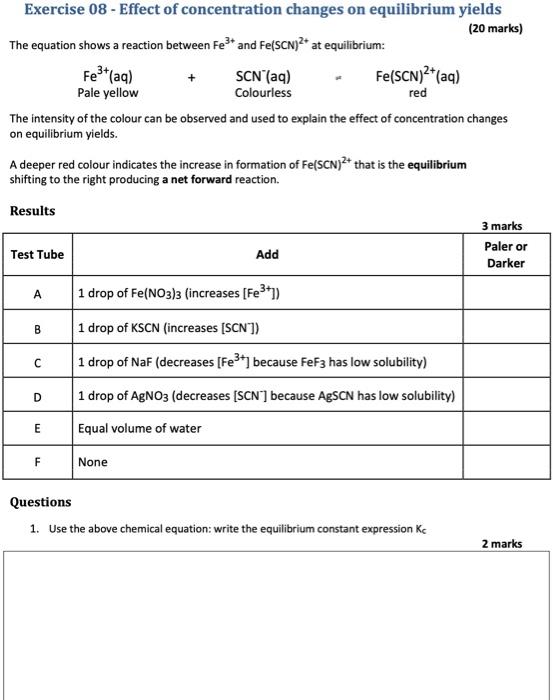

Exercise 08 - Effect of concentration changes on equilibrium yields (20 marks) The equation shows a reaction between Fe and Fe(SCN)2+ at equilibrium: Fe3+ (aq) SCN (aq) Fe(SCN)2+(aq) Pale yellow Colourless red The intensity of the colour can be observed and used to explain the effect of concentration changes on equilibrium yields. A deeper red colour indicates the increase in formation of Fe(SCN)2+ that is the equilibrium shifting to the right producing a net forward reaction. Results 3 marks Paler or Darker Test Tube Add A 1 drop of Fe(NO3)3 (increases [Fe3+1) 00 B 1 drop of KSCN (increases (SCN) D 1 drop of Naf (decreases [Fe3+] because FeF3 has low solubility) 1 drop of AgNO3 (decreases (SCN) because AgSCN has low solubility) Equal volume of water E F None Questions 1. Use the above chemical equation: write the equilibrium constant expression Kc 2 marks 2. Using +++++ (or - for no change) indicate the effects of changes in conditions on equilibrium and the concentration of the ions when the reagents are added relative to their starting concentrations. (Initial means the instant the reagent is added, Final means once the system has come to a new equilibrium. Assume a precipitate forms the instant a reagent is added) 10 marks Change in Fe Change in 'SCN Change in Fe(SCN) Shift in equilibrium Initial Final Initial Final Initial Final Forward or backward) A Test B D E 3. a) State Le Chatelier's Principle b) use it to explain the shift in equilibrium in Test A. 2 marks 4. Sketch a graph using different colours to show the concentration changes of each of (SCN) [Fe -1 [Fe(SCN2+1 in Test A (ignore the dilution factor of the drops) 3 marks Concentration -Initial Equilibrium Reagent Added Final Equilibrium
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


