Question: can you answer A please. show step by step work mol=MW(molg)mass(g)mass=volume(mL)density(g/mL)%yield=theoriticalexperimental100% 1. Consider the following Friedel crafts reaction and answer question from A-C A) What
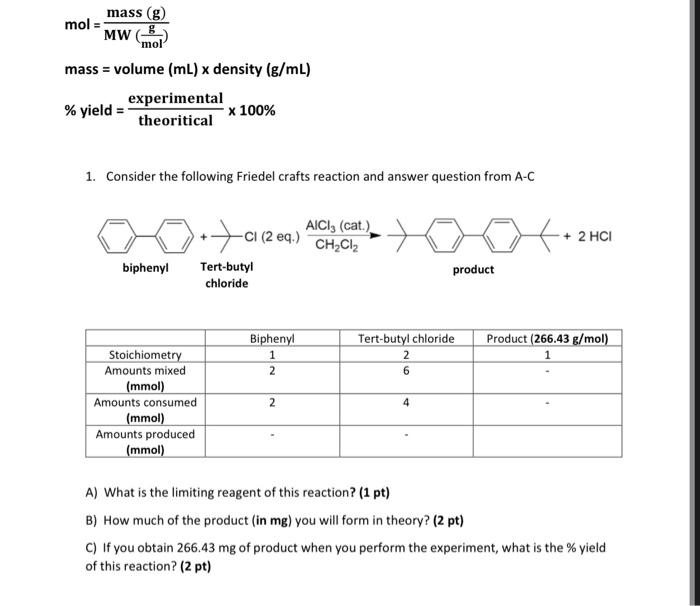
mol=MW(molg)mass(g)mass=volume(mL)density(g/mL)%yield=theoriticalexperimental100% 1. Consider the following Friedel crafts reaction and answer question from A-C A) What is the limiting reagent of this reaction? (1 pt) B) How much of the product (in mg ) you will form in theory? (2 pt) C) If you obtain 266.43mg of product when you perform the experiment, what is the \% yield of this reaction? (2 pt)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


