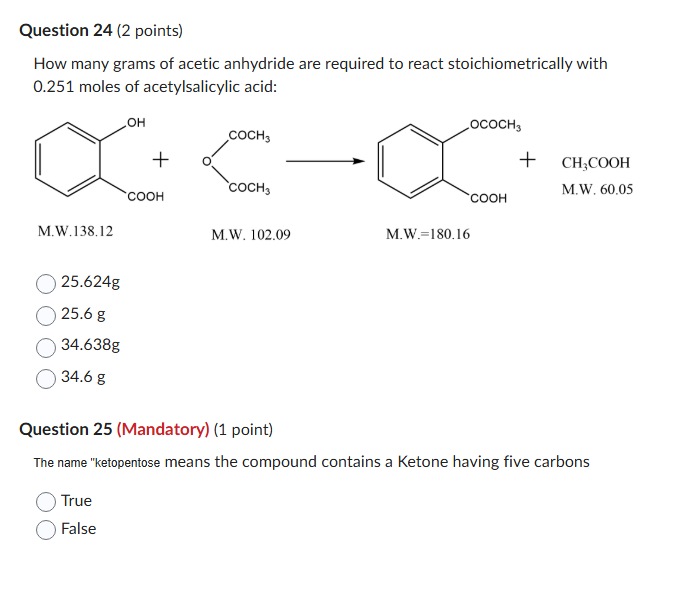
Question: can you answer question 24 and 25 Question 24 (2 points) How many grams of acetic anhydride are required to react stoichiometrically with 0.251 moles
can you answer question 24 and 25
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


