Question: can you answer this question please Q1: Stack gas from a furnace is used to dry rice. The flowsheet and known data are shown in
can you answer this question please
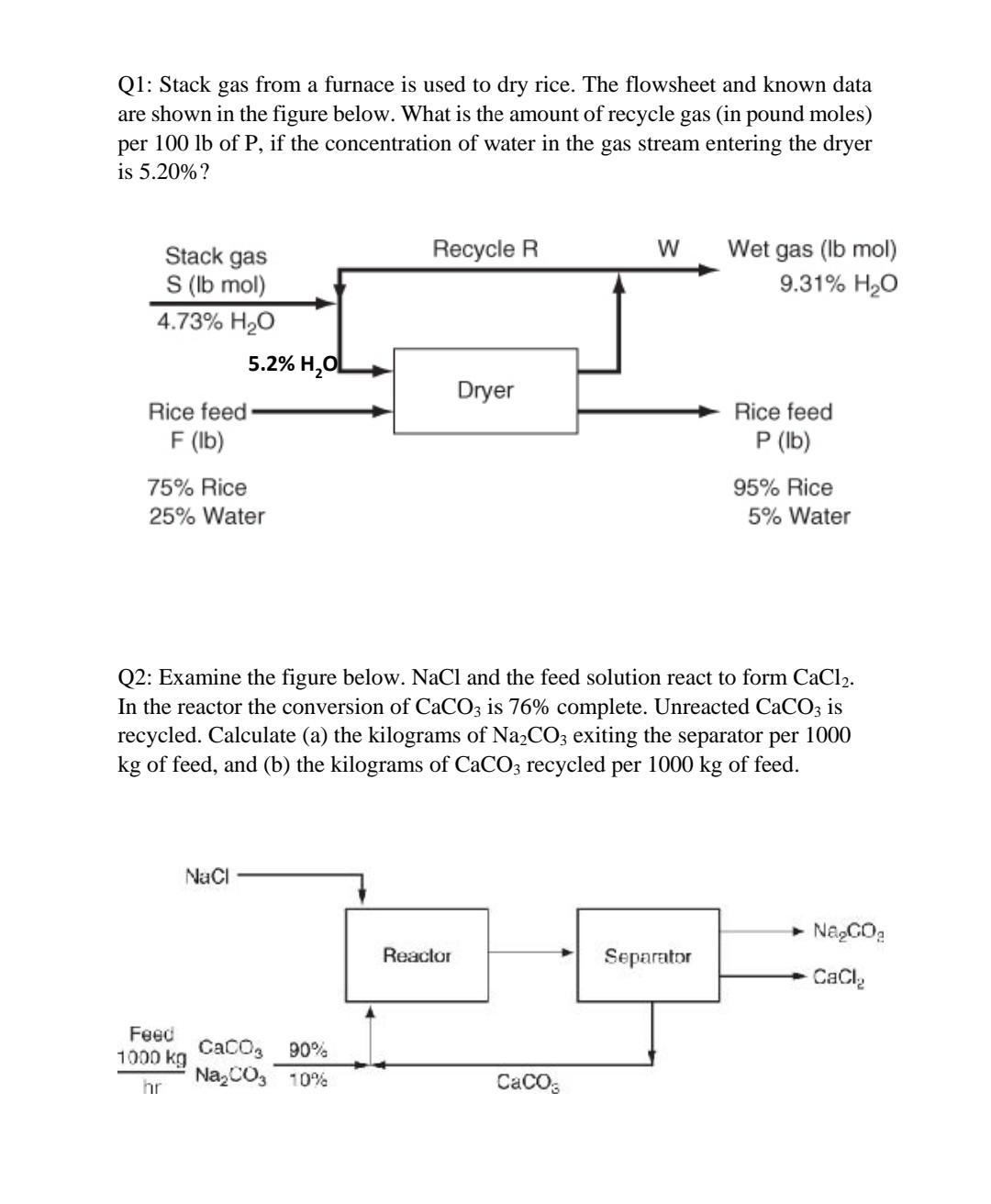
Q1: Stack gas from a furnace is used to dry rice. The flowsheet and known data are shown in the figure below. What is the amount of recycle gas (in pound moles) per 100 lb of P, if the concentration of water in the gas stream entering the dryer is 5.20%? Recycle R W Wet gas (lb mol) 9.31% H2O Stack gas S (lb mol) 4.73% H2O 5.2% H2OL Dryer Rice feed F(lb) 75% Rice 25% Water Rice feed P (lb) 95% Rice 5% Water Q2: Examine the figure below. NaCl and the feed solution react to form CaCl2. In the reactor the conversion of CaCO3 is 76% complete. Unreacted CaCO3 is recycled. Calculate (a) the kilograms of Na2CO3 exiting the separator per 1000 kg of feed, and (b) the kilograms of CaCO3 recycled per 1000 kg of feed. Naci Na,GO Reactor Separator Cacla Feed Cacos 90% 1000 kg Na,CO, 10% hr Cacos
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


