Question: can you guys please explain how the calculation was done especially how 800 was gotten and not 804 Example 23.11 The surface charge on a
can you guys please explain how the calculation was done especially how 800 was gotten and not 804
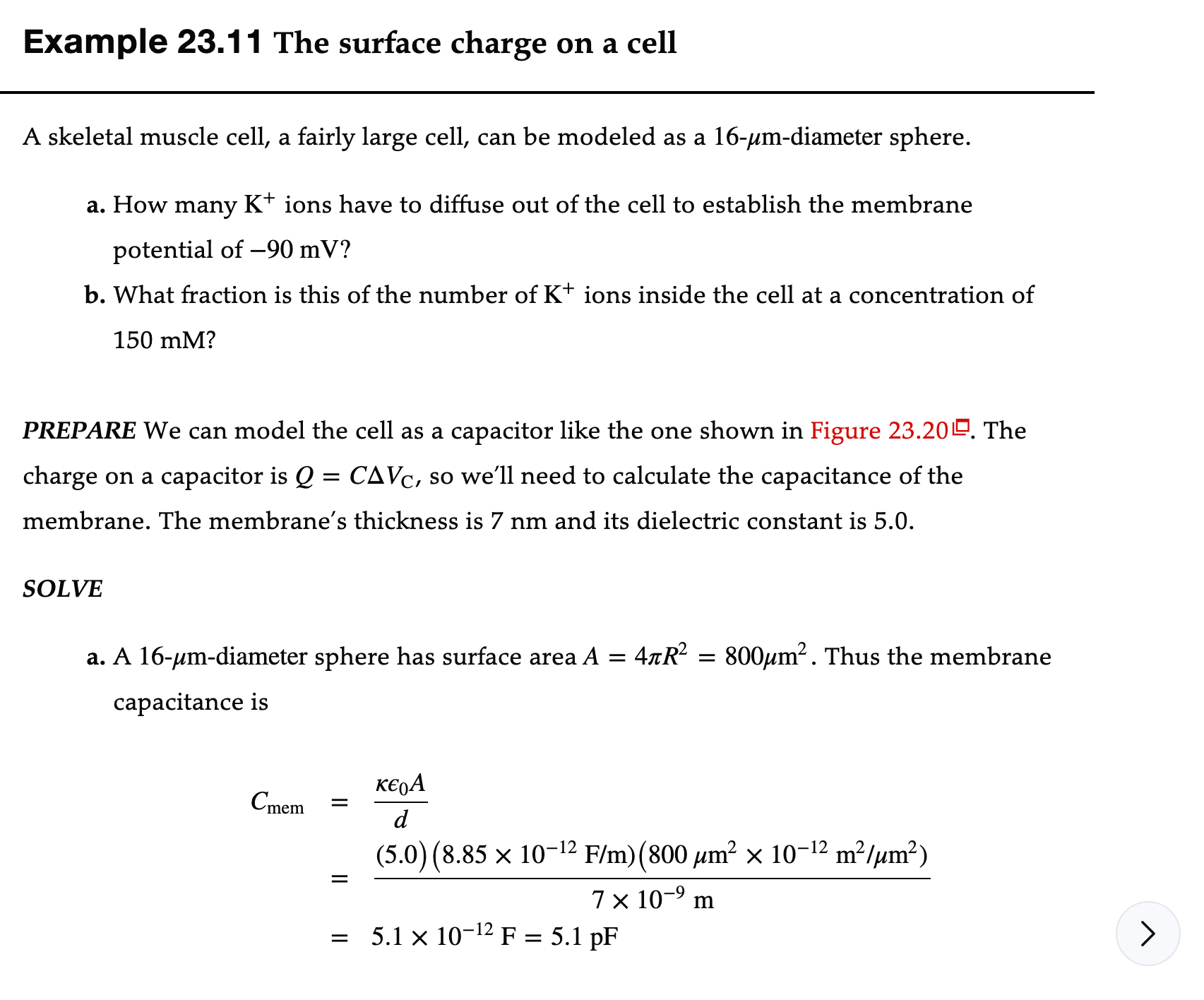


Example 23.11 The surface charge on a cell A skeletal muscle cell, a fairly large cell, can be modeled as a 16-ym-diameter sphere. a. How many K+ ions have to diffuse out of the cell to establish the membrane potential of 90 mV? b. What fraction is this of the number of K+ ions inside the cell at a concentration of 150 mM? PREPARE We can model the cell as a capacitor like the one shown in Figure 23.20 E'. The charge on a capacitor is Q = CAVC, so we'll need to calculate the capacitance of the membrane. The membrane's thickness is 7 nm and its dielectric constant is 5.0. SOLVE a. A 16-Mm-diameter sphere has surface areaA = 471'R2 = 800ym2. Thus the membrane capacitance is K6 A Cmem d0 (5.0) (8.85 x 10-12 F/m)(800 \"m2 x 10-12 m2/,um2) 7 X 10'9 m 5.1 x 10121: = 5.1pF Only one significant figure is justified because the membrane thickness is known to one significant figure. This is a very small amount of charge. The number of K+ surface ions needed to establish this charge is Nsurface = 2 = 3x 106 In other words, 3 x 10 K+ ions have to diffuse out of the cell and gather on the outer surface, leaving a surplus of 3 x 10 Cl- ions inside, to establish the -90 mV membrane potential. b. The number of K ions inside the cell is Ninside = Cin V. The cell volume is V = 2 TR' = 2100 um' . Here we do need to convert the concentration from molarity to SI units: 6.02 x 102 ions 1000 L c = 0.15 mol/L X X 1 mol 1 m-3 = 9.0 x 1025 ions/m3 Thus the number of K+ ions inside the cell is Ninside = Cin V = (9.0 x 1025 ions/m3) (2100 um3 x (10 6 m ) = 1.9 x 10 ions The number of ions that diffuse out as a fraction of the number of ions available isV = 5 HR\" = 2100 ,um'. Here we do need to convert the concentration from molarity to SI units: 6.02 x 1023 ' 1000 L c = 0.15 mol/L x 1ons X 1 mol 1 m3 9.0 x 1025 ions/m3 Thus the number of K+ ions inside the cell is Mnside 1 mm 3 ainv = (9.0 x 1025 ions/m3) (2100 \"m3 X (106 m) ) 1.9 x 1011 ions The number of ions that diffuse out as a fraction of the number of ions available is Nsurface = 3 X 106 z 2 X 105 Mnside 1.9 X 1011 ASSESS You might think that ions diffusing out of the cell would lower the ion concentration inside. It turns out that the number of ions needed on the exterior surface to establish the membrane potential is a tiny fraction of the number of available ions inside. The very slight decrease in concentration is not measurable, and the Nernst potential of potassium does not change due to this diffusion. Note that actual muscle cells are elongated, not spherical. Even so, a simple model of the cell as a sphere is adequate for doing one-signicantgure estimates. A cylindrical-cell model would be bit more complex, but it would not change the results
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


