Question: Can you help solve question 4 and 5 3. Baking soda (NaHCO3) thermally decomposes to soda ash (Na2CO3), CO2, and H20: 2 NaHCO3(s) Na2CO3(s) +
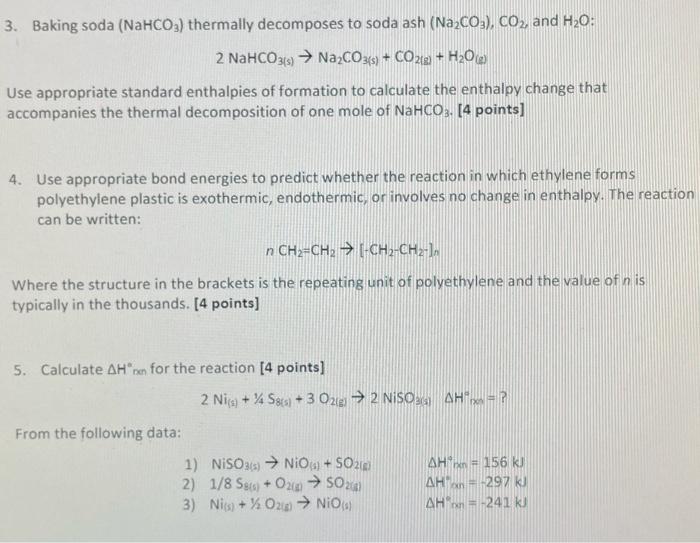
3. Baking soda (NaHCO3) thermally decomposes to soda ash (Na2CO3), CO2, and H20: 2 NaHCO3(s) Na2CO3(s) + CO2(a + H20 Use appropriate standard enthalpies of formation to calculate the enthalpy change that accompanies the thermal decomposition of one mole of NaHCO3- [4 points] 4. Use appropriate bond energies to predict whether the reaction in which ethylene forms polyethylene plastic is exothermic, endothermic, or involves no change in enthalpy. The reaction can be written: n CH2=CH2 [-CH2-CH2+) Where the structure in the brackets is the repeating unit of polyethylene and the value of n is typically in the thousands. [4 points] 5. Calculate An for the reaction [4 points) 2 Nis + % S8 +30210 2 NISO AHP From the following data: 1) NISO2) NO + SO20 AH = 156 K) 2) 1/8 S4 + 0212 5024) AH 297 kJ 3) Nis) + % 026) NO) AH-241 kJ
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


