Question: Can you please answer all the question? Consider a Carnot cycle as shown below for an atomic ideal gas in which the initial state is
Can you please answer all the question?
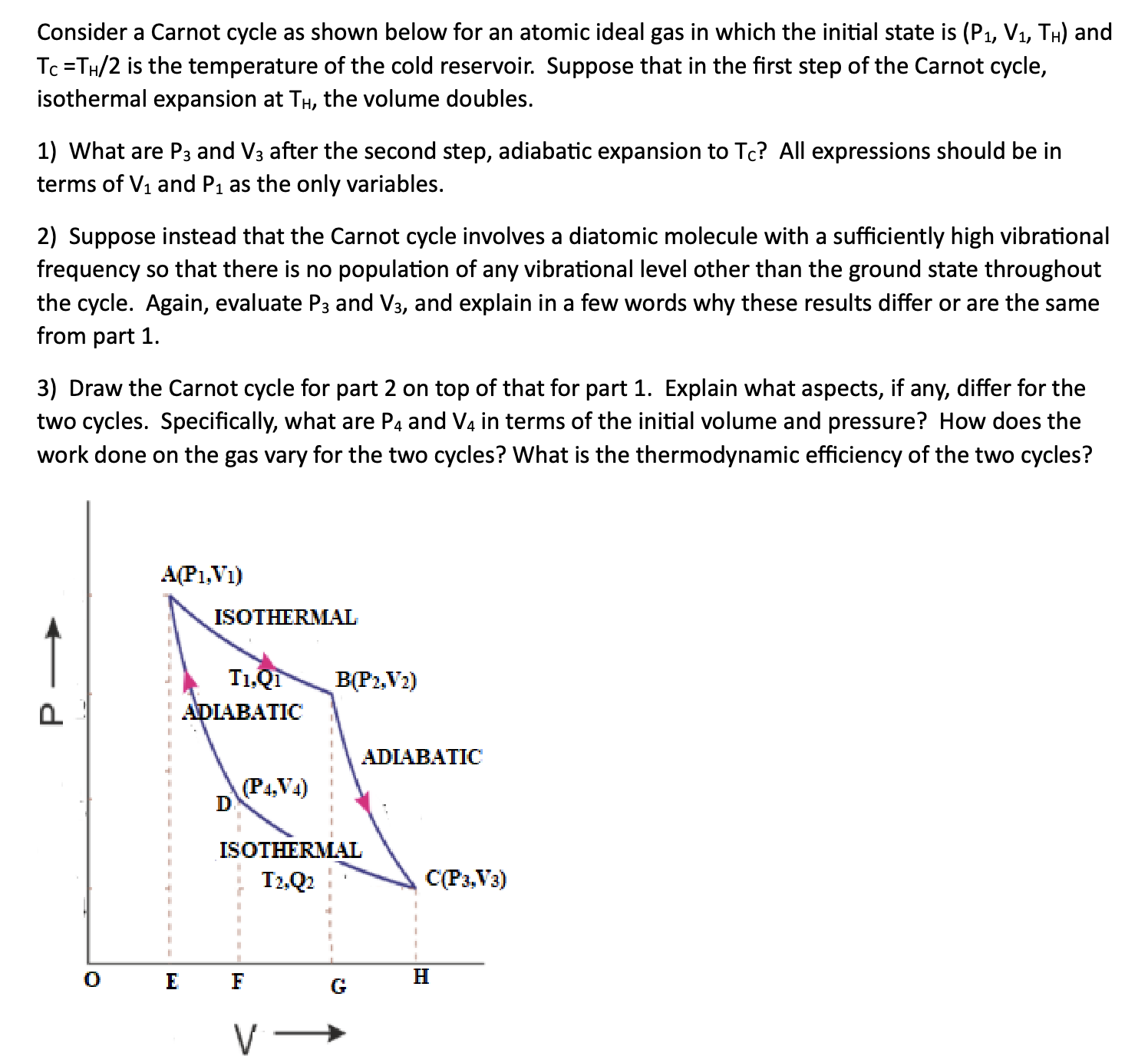
Consider a Carnot cycle as shown below for an atomic ideal gas in which the initial state is (P1, V1, TH) and To =TH/2 is the temperature of the cold reservoir. Suppose that in the first step of the Carnot cycle, isothermal expansion at TH, the volume doubles. 1) What are P3 and V3 after the second step, adiabatic expansion to Tc? All expressions should be in terms of V1 and P1 as the only variables. 2) Suppose instead that the Carnot cycle involves a diatomic molecule with a sufficiently high vibrational frequency so that there is no population of any vibrational level other than the ground state throughout the cycle. Again, evaluate P3 and V3, and explain in a few words why these results differ or are the same from part 1. 3) Draw the Carnot cycle for part 2 on top of that for part 1. Explain what aspects, if any, differ for the two cycles. Specifically, what are P4 and V4 in terms of the initial volume and pressure? How does the work done on the gas vary for the two cycles? What is the thermodynamic efficiency of the two cycles? A(P1,VI) ISOTHERMAL T1,Q1 B(P2,V2) Q ADIABATIC ADIABATIC D (P4, V4) ISOTHERMAL T2,Q2 C(Pa,Va) O E F G H V
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


