Question: can you please answer the discussion questions Data Analysis and Discussion 1. Discuss how the results of part A are used in successfully separating the

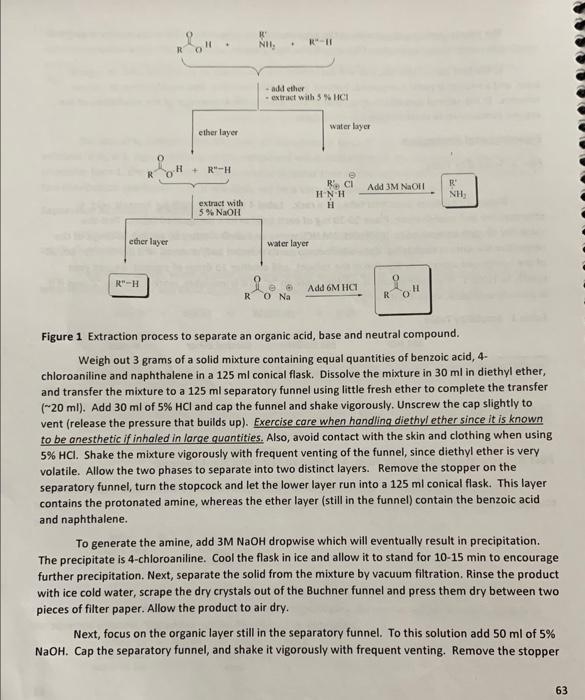

Data Analysis and Discussion 1. Discuss how the results of part A are used in successfully separating the three organic compounds in part B. 2. Calculate the composition of the unknown mixture in weight percent. 3. Evaluate the purity of each isolated product by comparing the observed melting point with the literature value. Obtain 12 medium-sized test tubes in a rack. Label 4 test tubes each for benzoic acid, 4chloroaniline and naphthalene. Place 1020mg of each solid in its 4 designated tubes. Test the solubility of each compound in 3MNaOH,3MHCl, water and diethyl ether by adding 10 drops of the respective solvents in each tube. Note which compounds dissolve and/or react. If a portion but not all of the compound appears to dissolve in the solvent, add another 10 drops of solvent. Record your results in Table 1. If the compound is soluble, mark an " s ", if it is insoluble, mark it with an "i" and if it is partially insoluble, mark it with a " p ". Table 1 Solubility Table Part B: Separation of Benzoic Acid, 4-Chloroaniline, and Naphthalene If you have an organic acid, a base, and a neutral compound in a mixture, you can separate the compounds by an acid-base extraction process (Figure 1). Weigh out 3 grams of a solid mixture containing equal quantities of benzoic acid, 4chloroaniline and naphthalene in a 125ml conical flask. Dissolve the mixture in 30ml in diethyl ether, and transfer the mixture to a 125ml separatory funnel using little fresh ether to complete the transfer (20ml). Add 30ml of 5%HCl and cap the funnel and shake vigorously. Unscrew the cap slightly to vent (release the pressure that builds up). Exercise care when handling diethyl ether since it is known to be anesthetic if inhaled in large quantities. Also, avoid contact with the skin and clothing when using 5%HCl. Shake the mixture vigorously with frequent venting of the funnel, since diethyl ether is very volatile. Allow the two phases to separate into two distinct layers. Remove the stopper on the separatory funnel, turn the stopcock and let the lower layer run into a 125ml conical flask. This layer contains the protonated amine, whereas the ether layer (still in the funnel) contain the benzoic acid and naphthalene. To generate the amine, add 3MNaOH dropwise which will eventually result in precipitation. The precipitate is 4-chloroaniline. Cool the flask in ice and allow it to stand for 1015min to encourage further precipitation. Next, separate the solid from the mixture by vacuum filtration. Rinse the product with ice cold water, scrape the dry crystals out of the Buchner funnel and press them dry between two pieces of filter paper. Allow the product to air dry. Next, focus on the organic layer still in the separatory funnel. To this solution add 50ml of 5% NaOH. Cap the separatory funnel, and shake it vigorously with frequent venting. Remove the stopper 10 Starting +wetght muss for 1:1:1 Benzoic acid: 0.987g 4 -chloro ahnine: 1.027g napha thatere: 1.009g 1st filtration Filtespuper:0.173dgcrestalsandpaper:0.533gStarting112Cend:123C crystals and paper: 0 crustals: 0.360g 2nd filtration watenyiuss94.316gfilerpuper:0.172gStart:63C7C fikr puper: 0.172g arter paper, erystals and vatingless. 45.110g crystass: 0.682g 3.d hifration watehgrass: 29260g filkpaperio.70gwatenglass,hiterpuperandStarts.70Cenos.77C 30 Cryslals.30.349 9 irustals: 0.967g
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


