Question: can you please help me solving these questions? Please provide reference if possible. POST-LAB QUESTIONS 1. On the basis of titration data, why might it

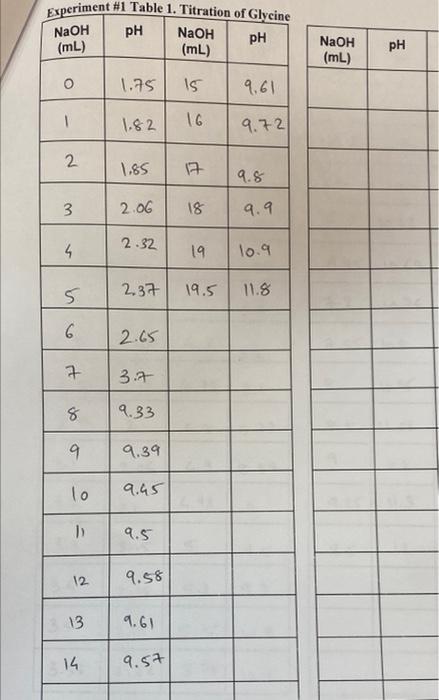
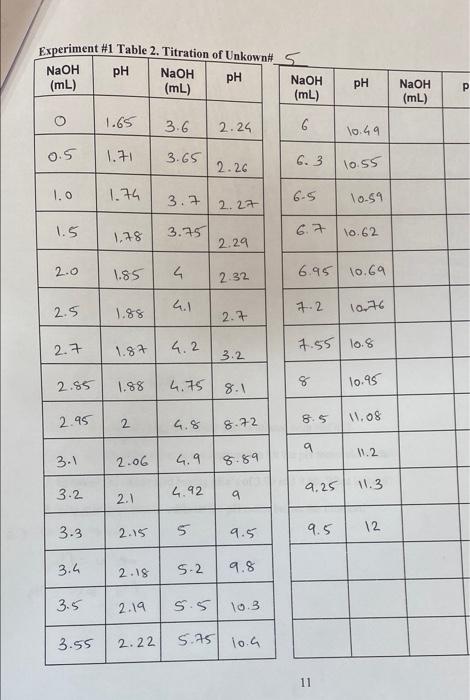
POST-LAB QUESTIONS 1. On the basis of titration data, why might it be difficult to distinguish between lysine and tyrosine? 2. What is the pH of each of these solutions? (a)10' M HNO3 (b) 6.21 x 10 * M HCI 3. Write the appropriate equilibrium (see Equation 5) for each pK, of glycine and of your unknown. 4. Define how many acid dissociation constants (pKa) a lysine has based on Table 1. How would you calculate the isoelectric point (pl) for lysine based on provided values? 5. The prevailing structure of the molecule in the one of the unknown amino acid solutions used in the experiment before titrating with NaOH is: COO- + H3 NC -H CH 3 What is the structure after the amino acid is titrated with NaOH? 6. Why the magnetic stirrer is not supposed to hit the pH meter electrode? 7. Consider the amino acid having pka's of 3.90 and 9.90. What is its pI? 8. Why it is important to calibrate the pH meter before the titration? Experiment #1 Table 1. Titration of Glycine pH NaOH pH (mL) NaOH (mL) NaOH (mL) PH O 1.75 15 9.61 1 1.82 16 9.72 2 1.85 7 9.8 3 2.06 18 . 2.32 4 19 10.9 5 2.37 19.5 11.8 6 2.65 P 3.7 8 9.33 9 9 9.39 lo 9.45 1) 9.5 12 9.58 13 9.61 14 9.57 Experiment #1 Table 2. Titration of Unkown# 5 NaOH pH NaOH (ml) (mL) NaOH (mL) pH pH NaOH (mL) O 1.65 3.6 2.26 6 10.49 OS 1.7 3.65 2.26 6.3 10.55 1.o 1.74 3.7 6.5 10.59 2.27 1.5 1.78 3.75 6.7 10.62 2.29 2.0 1.85 4 2.32 6.95 10.69 2.5 1.88 4.1 7.2 1076 2.7 2.7 1.87 4.2 1.55 10.8 3.2 2.85 op 1.88 4.75 8 10.95 8.1 2.95 2 85 1.08 6.8 8.72 q 1.2 3.1 2.06 6.9 8.89 9.25 11.3 3.2 4.92 2.1 N 9 3.3 2.15 5 5 9.5 12 9.5 3.h 2.18 5.2 9.8 3.5 2.19 5.5 10.3 3.55 2.22 5.75 10.a 11
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


