Question: can you please help me to solve this problem 1. The e.m.f of the following galvanic cell is 0.515 V at 25 C. Ag 9
can you please help me to solve this problem
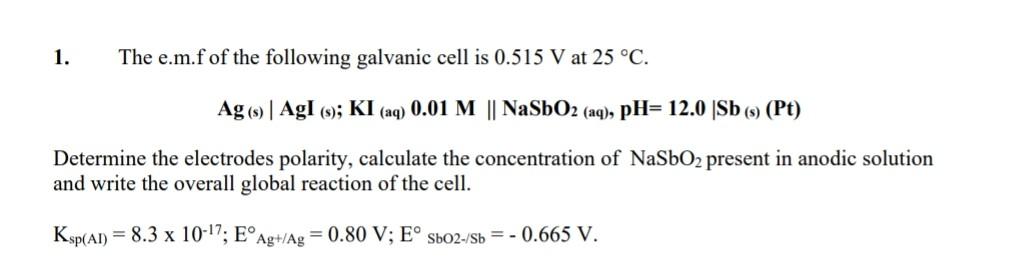
1. The e.m.f of the following galvanic cell is 0.515 V at 25 C. Ag 9 | Ag! (-); KI (aq) 0.01 M || NaSbO2 (aq), pH= 12.0 Sb ((Pt) 5) Determine the electrodes polarity, calculate the concentration of NaSbO2 present in anodic solution and write the overall global reaction of the cell. Ksp(A1) = 8.3 x 10-17; EAg+/Ag = 0.80 V; E Sb02-/Sb = -0.665 V
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


