Question: can you please help me with these two? 2) Complete the following Table that details the experimental parameters used to deduce the order with respect
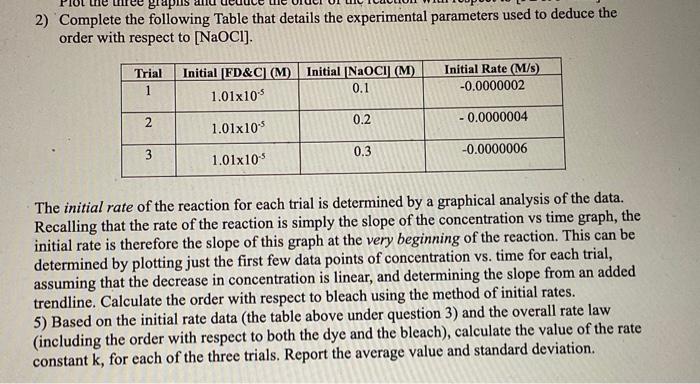
2) Complete the following Table that details the experimental parameters used to deduce the order with respect to [NaOCl]. The initial rate of the reaction for each trial is determined by a graphical analysis of the data. Recalling that the rate of the reaction is simply the slope of the concentration vs time graph, the initial rate is therefore the slope of this graph at the very beginning of the reaction. This can be determined by plotting just the first few data points of concentration vs. time for each trial, assuming that the decrease in concentration is linear, and determining the slope from an added trendline. Calculate the order with respect to bleach using the method of initial rates. 5) Based on the initial rate data (the table above under question 3) and the overall rate law (including the order with respect to both the dye and the bleach), calculate the value of the rate constant k, for each of the three trials. Report the average value and standard deviation
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


