Question: can you please help on how to solve these kind of modules b) [7.5] The reaction of FeCl2 and H20 (as stream 1 with flowrate
can you please help on how to solve these kind of modules
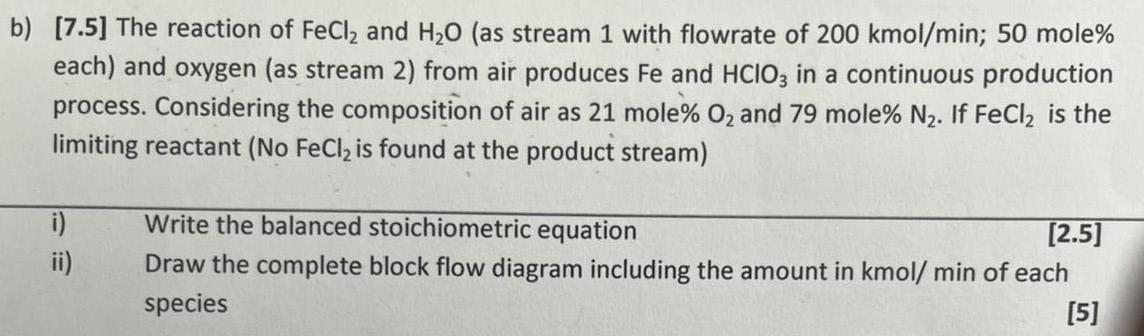
b) [7.5] The reaction of FeCl2 and H20 (as stream 1 with flowrate of 200 kmol/min; 50 mole% each) and oxygen (as stream 2) from air produces Fe and HCIO3 in a continuous production process. Considering the composition of air as 21 mole% O2 and 79 mole% N2. If FeCl2 is the limiting reactant (No FeCl2 is found at the product stream) i) ii) Write the balanced stoichiometric equation [2.5] Draw the complete block flow diagram including the amount in kmol/ min of each species [5]
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


