Question: Can you please order them from increasing boiling point, viscocity, freezing point, and vapor pressure of these molecules using the comparing method of their polarity,

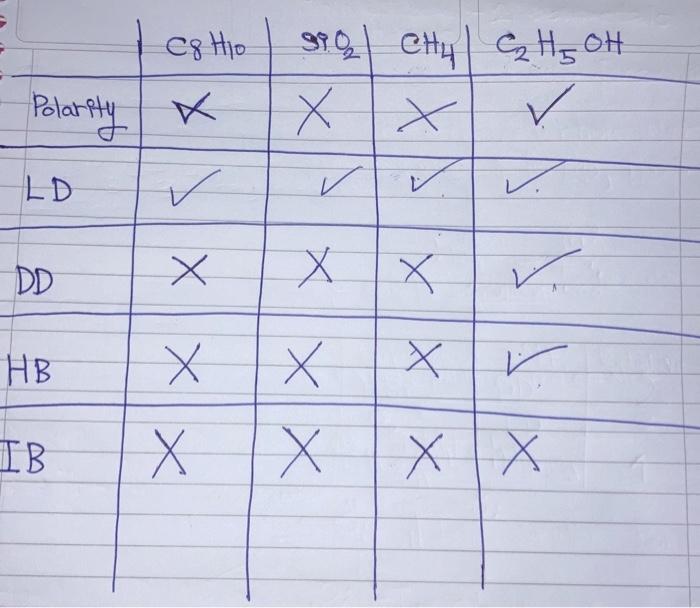
a naphthalene C.H. (Organic Solid) c. quartz SiO2 (Sand with crystalline structure) - Highest melting point b. methane CH4 (Gas) d. ethanol C,H,OH (Liquid Alcohol) C8 Ho Polarity 870) CH4 H, OH XX . s. LD . IX DD X XV. HB X x v XX IB X XX
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


